
(a)
Interpretation:
The order of the reaction with respect to substance
(a)
Explanation of Solution
The rate law can be represented as given below.
Where,
Carefully examining the table, it can be said that
By taking logarithm on both the sides, it can be written as follows,
The above equation is in the form of
Similarly, by keeping
By taking logarithm on both the sides, it can be written as follows,
The above equation is in the form of
Four data sets have constant
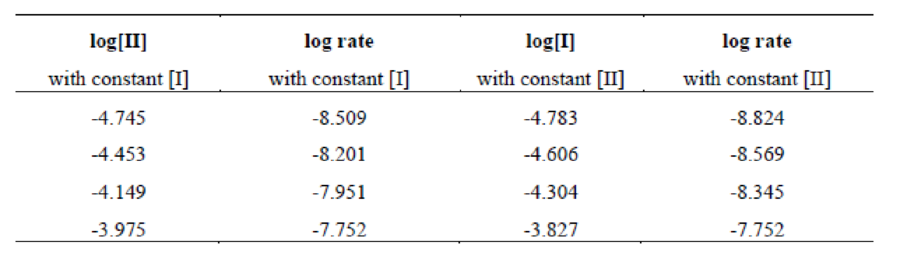
Figure 1
The graph of
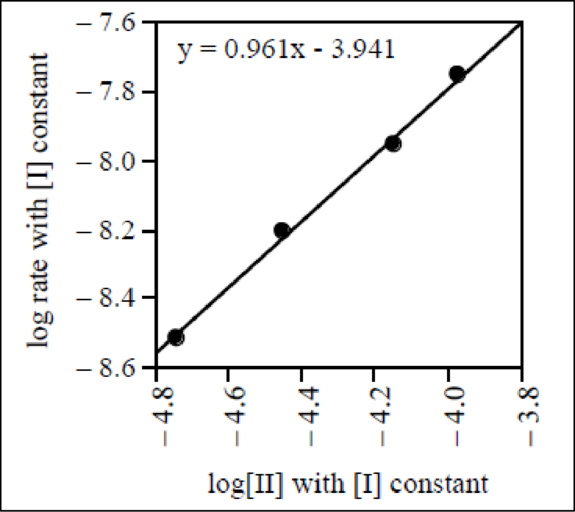
Figure 2
Therefore, the reaction order of reactant
The graph of
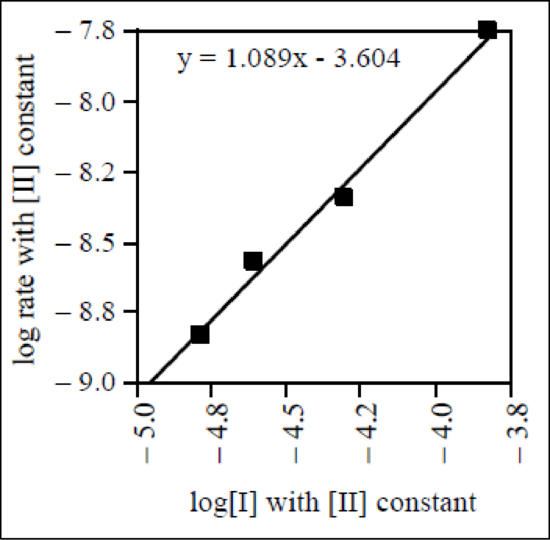
Figure 3
Therefore, the reaction order of reactant
(b)
Interpretation:
The rate law for the reaction has to be derived.
(b)
Explanation of Solution
The order of the reaction with respect to substance
(c)
Interpretation:
The rate constant
(c)
Answer to Problem 25QRT
The average of the rate constant is
Explanation of Solution
The rate constant can be expressed as shown below.
For first experiment the rate constant can be calculated by plugging all the data given in the table.
In first experiment, the initial concentration of
Now, the rate constant for first experiment is given below.
Similarly, the rate constant for rest of the experiments can be calculated. The calculated rate constant values are given in the table below.
|
Initial rate |
Rate constant | ||
The average of the rate constant is
(d)
Interpretation:
The initial rate of the reaction has to be calculated.
(d)
Answer to Problem 25QRT
The initial
Explanation of Solution
The rate law can be expressed as shown below.
Given that,
Therefore, the initial rate of reaction is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: The Molecular Science
- What is the difference between order of reaction and molecularity ?arrow_forwardWrite the mechanism of the reactionarrow_forwardDescribe the reaction mechanism for the hydrolysis of 2-bromo-2-methylpropane [CH3C(CH3)2Br] with aqueous hydroxide ions (OH-).The description should be detailed and must include the type of bond fission that takes place. You may sketch and insert suitable diagrams to aid your description if you wisharrow_forward
- it is known that a drug has a first order elimination rate constant of 0.17 h-1. If this drug is administered via a constant rate IV infusion, how long does it take for the plasma drug concentration to reach a steady state?arrow_forwardDerive an equation for the steady-state rate of the sequence of reactions A⇌B⇌C⇌D, with [A] maintained at a fixed value and the product D removed as soon as it is formed.arrow_forwardA certain first-order reaction has a rate constant of 1.50×10−3 s−1s−1. How long will it take for the reactant concentration to drop to 1818 of its initial value? Express your answer with the appropriate units.arrow_forward
- Calculate the rate constant of this reactionarrow_forwardIf 0.70 g of FeCl3 is used to iodinate 8.42 g of benzene with sufficient ICl, what is the average number of reactions that each FeCl3 molecule must catalyze?arrow_forwardIn the hydrogenation of ethylene using a nickel catalyst, the initial concentration of ethylene is 1.70 mol⋅L^−1 and its rate constant (k) is 0.0010 mol⋅L^−1⋅s^−1 . Determine the rate of reaction if it follows a zero-order reaction mechanism.arrow_forward
- calculate the rate constant (k) of the reactionarrow_forwardIn aqueous solution buffered at a pH of 6.75 Penicillin G (molecular weight of 334.39 g/mole) undergoes hydrolysis via a pseudo first order reaction that has an activation energy of 77.3 kJ/mole and a pseudo first order rate constant of 1.58 x 10-7 s-1 at 30°C. The solubility of the sodium salt of Penicillin G is 25 mg/ml of water. A solution containing the sodium salt of Penicillin G is determined to have a concentration of 1.25 x 10-3 M. The solution is… a. unsaturated b. saturated c. supersaturatedarrow_forwardExpress the rates of the following equation in terms of reactant and product. PCl₅↔ PClз + Cl₂arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

