
(a)
Interpretation:
The complete rate law for the hydrolysis of benzene sulfonyl chloride in presence of fluoride ion has to be derived.
(a)
Explanation of Solution
The rate is affected by the concentration of Benzenesulfonyl chloride
Where
In order to study the effect of
Therefore the adjusted rate law can be written in the following form.
Adjusted rate
The experiments
It is clear that the concentration change and the adjusted rate change are almost equal, so the fluoride ion concentration and adjusted rate are proportional. Though it is only an approximate relationship, a graph can be drawn by taking log of the rate on y axis and
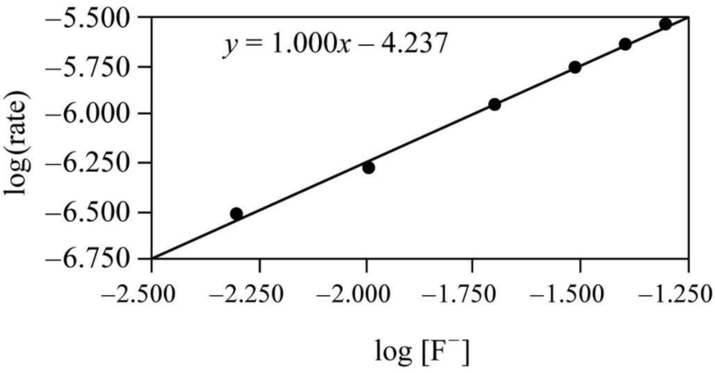
Figure 1
Therefore it is clear that the reaction is first order with respect to fluoride ion in the adjusted rate law.
So the rate can be written s follows,
(b)
Interpretation:
The rate constant has to be calculated for the hydrolysis of benzene sulfonyl chloride in presence of fluoride ion.
(b)
Explanation of Solution
The rate constant can be determined using the adjusted rate law as follows,
The rate constant can be calculated for the all the experiments with non-zero fluoride ion can be calculated as follows,
Therefore the average value of the rate constant is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: The Molecular Science
- The acid-catalyzed iodination of acetone CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + HI(aq) is a common laboratory experiment used in general chemistry courses to teach the method of initial rates. The reaction is followed spectrophotometrically by the disappearance of the color of iodine in the solution. The following data (J. P. Birk and D. L Walters, Journal of Chemical Education, Vol. 69, p. 585, 1992) were collected at 23 C for this reaction. Determine the rate law for this reaction.arrow_forwardList at least four experimentally determined parameters that you, an experimenter, can define when exploring the hydrolysis of ethyl benzoate by aqueous sodium hydroxide.arrow_forwardWhen enzymes are present at very low concentration, their effect on reaction rate can be described by first-order kinetics. Calculate by what factor the rate of an enzyme-catalyzed reaction changes when the enzyme concentration is changed from 1.5 107 M to 4.5 106 M.arrow_forward
- Derive the corresponding expression for t for a second-order reaction in terms of the initial concentration and rate constant. What is the relationship between t and t1/2 in this case?arrow_forwardA first-order decomposition reaction has a rate constant of 0.00530 yr−1. How long does it take for [reactant] to reach 12.5% of its original value? Be sure to report your answer to the correct number of significant figures.arrow_forwardA certain first-order reaction has a rate constant of 1.60×10−3 s−1. How long will it take for the reactant concentration to drop to 18 of its initial value?arrow_forward
- For a first order reaction R P, values of the rate constantarrow_forwardthe rate law for a reaction allows the speed of a reaction to berelated to the concentrations of reacting species, and also provides important informationabout the mechanism and activation energy. In this case, the reaction to be studied is theiodination of acetone in the presence of an acid catalyst.I2 (aq) + CH3COCH3 (aq) HI (aq) + ICH2COCH3 (aq)Many methods are possible to track the progress of a reaction, often relying on someform of spectroscopy. Conveniently for us, iodine in solution has a yellow-brown color,whereas the acetone and both products are colorless. This means that we can run the reactionusing iodine as the limiting reagent, and when we see that the yellow-brown color of the iodinehas disappeared, we know that the reaction has stopped and all of the iodine has beenconsumed.So how does this help us? Recall that to calculate a rate of reaction, we would like toknow the change in concentration of a reactant or product during a fixed amount of time. Wewill choose the…arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





