
Concept explainers
Give an acceptable IUPAC name for each of the following compounds:
Interpretation:
The acceptable IUPAC name for each of the given compounds is to be deteremined.
Concept introduction:
Alkanes have the general formula
The parent chain in each of hydrocarbon structure is first identified and the chain is numbered such that the substituents attached get lowest numbers.
Prefixes are used to denote the number of identical substituents.
The substituents are written in alphabetical order while writing the IUPAC name.
If the two groups of atoms that are the higher priority are on the same side of the double bond, then the bond is termed as the Z-configuration. While if the two group of higher priority are on the opposite side then the configuration is E-configuration.
Answer to Problem 27P
Solution:
a)
b)
c)
d)
e)
f)
g)
h)
Explanation of Solution
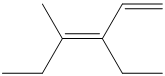
a) The expanded structure for the given structure can be drawn as follows:
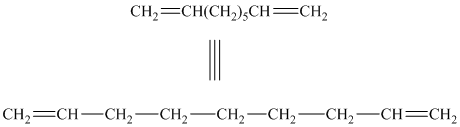
In the above structure, the parent chain is the continuous chain from
Thus, the IUPAC name is
b) The structure for the given compound is as follows:
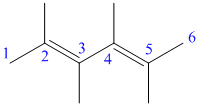
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
c) The structure for the given compound and its expanded structure is as follows:
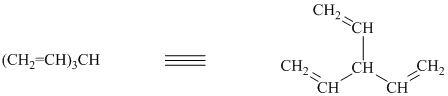
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
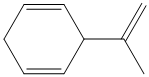
d) The structure for the given compound is as follows:
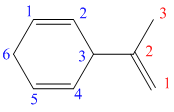
In the above structure, the parent ring contains six carbon atoms, so the parent name of the cycloalkane is cyclohexane. There are two double bonds in the given compound, the first one exist in between
Considering the Cahn–Ingold–Prelog sequence rules, for the cyclohexadiene, the higher priority groups are on the same side in both the double bonds. Hence, both get the stereochemical label as Z.
Thus, the IUPAC name of this structure is
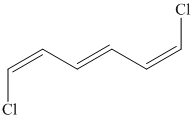
e) The structure for the given compound is as follows:

In the above structure, the parent longest chain is from
For double bonds at
For the internal double bond at carbon
Thus, the IUPAC name is
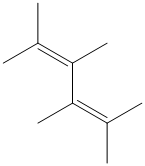
f) The structure for the given compound is as follows:
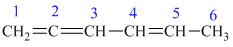
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
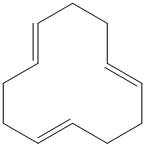
g) The structure for the given compound is as follows:
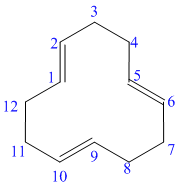
In the above structure, the parent ring contains twelve carbon atoms, so the parent name of the cycloalkane is cyclododecane. There are three double bonds in the given compound, the first one exist in between
For all the three double bonds at
Thus, the IUPAC name of this structure is
h) The structure for the given compound is as follows:
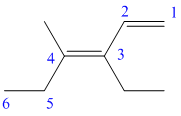
In the above structure, the parent longest chain is from
For the double bonds at
Thus, the IUPAC name is
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry - Standalone book
- Give the complete IUPAC name for each of the following compounds: (a) CH3CH2CBr2CH3 (b) (CH3)3CCl (c) (d) CH3CH2CCHCH3CH2CCH (e) (f) (g) (CH3)2CHCH2CH=CH2arrow_forwardIsooctane is the common name of the isomer of C8H18 used as the standard of 100 for the gasoline octane rating: (a) What is the IUPAC name for the compound? (b) Name the other isomers that contain a five-carbon chain with three methyl substituents.arrow_forwardWhat is the IUPAC name for the following compounds in the image. Please explain if it is cis or trans and illustrate the compounds in their corresponding drawings. For compound 1: a. cis-4-isopropylcyclohexanol b. trans-4-hydroxy-1-isopropylcyclohexane c. trans-4-isopropylcyclohexanol d. cis-4-hydroxy-1-isopropylcyclohexane e. p-isopropylcyclohexanol f. cis-1-hydroxy-4-isopropylcyclohexane For compound 2: a. 3-ethylpent-3-ol b. 2,2-diethylbut-1-ol c. 2-ethylpent-3-ol d. 2-ethylpent-2-olarrow_forward
- (a) Write the IUPAC names of the following compounds :(i) CH3CO(CH2)4CH3 (ii) Ph — CH = CH — CHO(b) Describe the following conversions in not more than two steps :(i) Ethanol to 3-Hydroxybutanal (ii) Benzoic acid to m-Nitrobenzyl alcohol(iii) Propanone to Propenearrow_forwardA2 1. An alkyne with molecular formula C5H10 2. A ketone with molecular formula C4H8O 3. A ketone with molecular formula C3H8O 4. An alkene with molecular formula C5H8 5. An alkene with molecular formula C5H10 6. An aldehyde with molecular formula C2H4O 7. An aldehyde with molecular formula CH4O 8. A saturated hydrocarbon with molecular formula C6H14arrow_forward1.Name the following compounds using the IUPAC Nomenclature. a. CH3CH(CH3)(CH2)4CH3 b. CH3 │ CH3-CH-CH-CH3 │ CH3 2. Draw the structures of each of the following compounds a. cis-1,2-Dichlorocyclopropane b.trans-1,4-Diethylcyclohexanearrow_forward
- Draw all the alkyl halides with molecular formula C 5H 11Cl formed when pentane (CH 3CH 2CH 2CH 2CH 3) is heated with Cl 2.arrow_forwardGive iupac name for this diol CH3CH(OH)(CH2)4CH(OH)C(CH3)3arrow_forwardGive the chemical name 1. CH3 - CH - CH - CH2 - CH - CH3 2. CH3-CH(CH3)-CH2-CH=C(CH2)(CH3)-CH(CH2)(CH3)-CH(CH2)(CH3)-CH2(CH3)arrow_forward
- Draw three constitutional isomers having molecular formula C 7H 14 that contain a fi ve-membered ring and two CH 3 groups bonded to the ring. Give the IUPAC name for each isomerarrow_forwardIdentify the substituents and the systematic name of the compound.arrow_forwardWhat is the correct IUPAC name for the following structures: CH3COOCH3 CH3-CH2-CO-CH3arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


