
Consider a two-stage compression refrigeration system operating between the pressure limits of 1.4 and 0.12 MPa. The working fluid is refrigerant-134a. The refrigerant leaves the condenser as a saturated liquid and is throttled to a flash chamber operating at 0.5 MPa. Part of the refrigerant evaporates during this flashing process, and this vapor is mixed with the refrigerant leaving the low-pressure compressor. The mixture is then compressed to the condenser pressure by the high-pressure compressor. The liquid in the flash chamber is throttled to the evaporator pressure, and it cools the refrigerated space as it vaporizes in the evaporator. Assuming the refrigerant leaves the evaporator as saturated vapor and both compressors are isentropic, determine (a) the fraction of the refrigerant that evaporates as it is throttled to the flash chamber, (b) the amount of heat removed from the refrigerated space and the compressor work per unit mass of refrigerant flowing through the condenser, and (c) the coefficient of performance.
(a)
The fraction of the refrigerant that evaporates as it is throttled to the flash chamber.
Answer to Problem 113RP
The fraction of the refrigerant that evaporates as it is throttled to the flash chamber is
Explanation of Solution
Show the T-s diagram as in Figure (1).
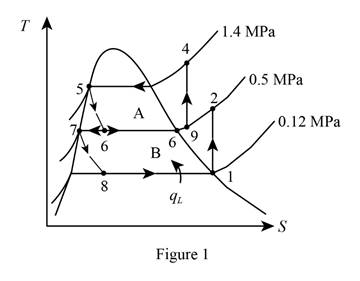
From Figure (1), write the specific enthalpy at state 5 is equal to state 6 due to throttling process.
Here, specific enthalpy at state 5 and 6 is
From Figure (1), write the specific enthalpy at state 7 is equal to state 8 due to throttling process.
Here, specific enthalpy at state 7 and 8 is
Express the fraction of the refrigerant that evaporates as it is throttled to the flash chamber
Here, specific enthalpy at saturated vapor is
Conclusion:
Perform unit conversion of pressure at state 1 from
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 1
Here, specific entropy and enthalpy at state 1 is
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2 corresponding to pressure at state 2 of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy at state 2 and specific enthalpy at state 2 respectively.
Show the specific enthalpy at state 2 corresponding to specific entropy as in Table (1).
|
Specific entropy at state 2 |
Specific enthalpy at state 2 |
| 0.9384 | 263.48 |
| 0.94789 | |
| 0.9704 | 273.03 |
Substitute
Thus, the specific enthalpy at state 2 is,
Perform unit conversion of pressure at state 3 from
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 3
Perform unit conversion of pressure at state 5 from
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 5
Here, specific enthalpy at saturated liquid is
Substitute
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 8
Substitute
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the specific enthalpy at evaporation and pressure of
Substitute
Hence, the fraction of the refrigerant that evaporates as it is throttled to the flash chamber is
(b)
The amount of heat removed from the refrigerated space and the compressor work per unit mass of refrigerant flowing through the condenser.
Answer to Problem 113RP
The amount of heat removed from the refrigerated space is
Explanation of Solution
Express the enthalpy at state 9 by using an energy balance on the mixing chamber.
Here, the rate of total energy entering the system is
Express the amount of heat removed from the refrigerated space.
Express the compressor work per unit mass of refrigerant flowing through the condenser.
Conclusion:
Substitute
Refer Table A-13, “superheated refrigerant 134a”, and write the specific entropy at state 9 corresponding to pressure at state 9 of
Show the specific enthropy at state 9 corresponding to specific enthalpy as in Table (2).
|
Specific enthalpy at state 9 |
Specific entropy at state 9 |
| 263.48 | 0.9384 |
| 264.28 | |
| 273.03 | 0.9704 |
Use excels and tabulates the values of Table (2) in Equation (IV) to get,
Thus, the specific entropy at state 9 is,
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 4 corresponding to pressure at state 4 of
Substitute
Hence, the amount of heat removed from the refrigerated space is
Substitute
Hence, the compressor work per unit mass of refrigerant flowing through the condenser is
(c)
The coefficient of performance of the system.
Answer to Problem 113RP
The coefficient of performance of the system is
Explanation of Solution
Express the coefficient of performance of the system.
Conclusion:
Substitute
Hence, the coefficient of performance of the system is
Want to see more full solutions like this?
Chapter 11 Solutions
Thermodynamics: An Engineering Approach
- When a standard-efficiency air-cooled condenser is used, the condensing refrigerant will normally be higher in temperature than the entering air temperature.arrow_forwardWhat are the approximate temperature ranges tor low-, medium-, and high-temperature refrigeration applications?arrow_forwardWhy is truck refrigeration designed to provide low- and medium-temperature refrigeration?arrow_forward
- How will oversizing the buffer tank on a water-to-water heat pump system affect the run time of the system?arrow_forwardConsider a two-stage cascade refrigeration system operating between the pressure limits of 0.8 and 0.14 MPa. Each stage operates on the ideal vapor-compression refrigeration cycle with refrigerant-134a as the working fluid. Heat rejection from the lower cycle to the upper cycle takes place in an adiabatic counterflow heat exchanger where both streams enter at about 0.4 MPa. If the mass flow rate of the Refrigerant through the upper circle is 0.24 kg/s, determine (a)the mass flow rate of the refrigerant through the lower cycle, (b)the rate of heat removal from the refrigerated space and the power input to the compressor, and (c)the coefficient of performance of this cascade refrigerator. Answers: (a) 0.195 kg/s, (b) 34.2 kW, 7.63 kW, (c) 4.49arrow_forward1. Consider a two-stage cascade refrigeration system operating between the pressure limits of 1.2 MPa and 200 kPa with refrigerant-134a as the working fluid.Heat rejection from the lower to the upper cycle takes place in a countercurrent adiabatic heat exchanger where the pressures in the upper and lower cycles are 0.4 and 0.5 MPa, respectively. In both cycles the refrigerant is a saturated liquid at the condenser outlet and a saturated vapor at the compressor inlet, and the isentropic efficiency of the compressor is 80 percent.The mass flow rate of the refrigerant in the lower cycle is 0.15 kg/s.Determine the following:a) the mass flow of the refrigerant through the upper cycle,b) the rate of removal from the refrigerated space andc) the COP of this refrigerator.d) Draw the T-s graph of the complete system and the diagram with all the components and processes of both cycles, indicate all the states in both cases (1, 2, 3….)arrow_forward
- A two-stage compression refrigeration system operates with refrigerant-134a between the pressure limits of 1.4 MPa and 0.10 MPa. The refrigerant leaves the condenser as a saturated liquid and is throttled to a flash chamber operating at 0.6 MPa. he flash chamber is maintained at the same pressure as the low pressure discharge which is 0.6 Mpa. The vapor in the flash chamber is then compressed to the condenser pressure by the high-pressure compressor, and the liquid is throttled to the evaporator pressure. Assume the refrigerant leaves the evaporator as saturated vapor and both compressors are isentropic. Consider a mass flow rate of 0.19 kg/s through the condenser. (Take the required values from saturated refrigerant-134a tables.) Determine the fraction of the refrigerant that evaporates as it is throttled to the flash chamber. (You must provide an answer before moving to the next part.) The fraction of the refrigerant that evaporates as it is throttled to the flash chamber is…arrow_forwardA two-stage compression refrigeration system operates with refrigerant-134a between the pressure limits of 1.4 MPa and 0.10 MPa. The refrigerant leaves the condenser as a saturated liquid and is throttled to a flash chamber operating at 0.6 MPa. he flash chamber is maintained at the same pressure as the low pressure discharge which is 0.6 Mpa. The vapor in the flash chamber is then compressed to the condenser pressure by the high-pressure compressor, and the liquid is throttled to the evaporator pressure. Assume the refrigerant leaves the evaporator as saturated vapor and both compressors are isentropic. Consider a mass flow rate of 0.19 kg/s through the condenser. (Take the required values from saturated refrigerant-134a tables.) Determine the coefficient of performance. The coefficient of performance is ______.arrow_forwardDetermine the degrees of subcooling at the exit of the condenser of a 2-ton air-conditioner system. The system operates on the ideal, vapor-compression refrigerationcycle with the following design parameters: R-134a flow rate 0.05 kg/s Evaporator Pressure 200 kPa Condenser Pressure 1200 kPaarrow_forward
- Consider a two-stage cascade refrigeration system operating between the pressure limits of 0.8 and 0.14 MPa. Each stage operates on the ideal vapor-compression refrigeration cycle with refrigerant-134a as the working fluid. Heat rejection from the lower cycle to the upper cycle takes place in an adiabatic counter-flow heat exchanger where both streams enter at about 0.4 MPa. If the mass flow rate of the refrigerant through the upper cycle is 0.24 kg/s, determine (a) the mass flow rate of the refrigerant through the lower cycle, (b) the rate of heat removal from the refrigerated space and the power input to the compressor, (c) the coefficient of performance of this cascade refrigerator, and (d) the intermediate pressure of the cascade cycle. For full credit, accurately, neatly draw the cascade system on an electronic PDF p-h diagram. Please only solve part d. If possible, show the ph diagramarrow_forwardConsider a two-stage cascade refrigeration system operating between the pressure limits of 2 MPa and 0.05 MPa. Each stage operates on an ideal vapor-compression refrigeration cycle with R-134a as the working fluid. Heat rejection from the lower cycle to the upper cycle takes place in an adiabatic counterflow heat exchanger where both streams enter at 0.5 MPa. If the mass flow rate of the refrigerant through the upper cycle is 0.25 kg/s, What would the COP be if the heat exchanger pressure were 0.7 MPa. Group of answer choices 2.50 1.50 1.48 1.51arrow_forwardConsider a refrigrator that operates on the vapor compression refrigeration cycle with R-134a as the working fluid. The refrigerant enters the compressor as saturated vapor at 70 kPa, and exits at 1200 kPa and 90°C, and leaves the condenser as saturated liquid at 1200 kPa. The coefficient of performance of this refrigrator isarrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
