
The greatest amount of exergy destroyed among the processes of system.
Answer to Problem 117RP
The greatest amount of exergy destroyed among the processes of system is
Explanation of Solution
Sketch the schematic layout of the two-stage compression refrigeration system as in Figure (1).
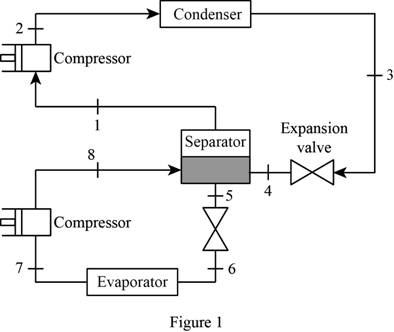
Sketch the
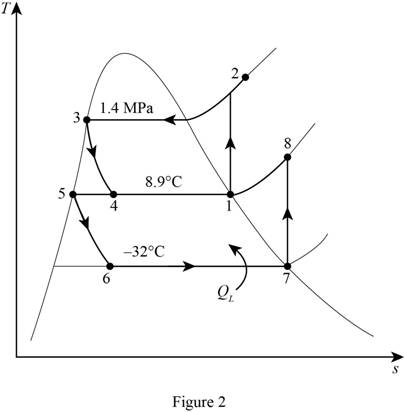
Write the relation between the specific enthalpies at the inlet and exit of throttling.
Here, specific enthalpy of the refrigerant at the inlet of throttling is
Write the energy balance equation for the separator.
Here, mass flow rate of the refrigerant at the end of high pressure compressor is
Write the expression to calculate the quality of the refrigerant
Here, specific enthalpy of the saturated liquid is
Write the expression to calculate the specific entropy for saturated refrigerant
Here, specific entropy of the saturated liquid is
Write the expression to calculate the rate of cooling produced
Here, specific enthalpy at the inlet of low pressure compressor is
Write the expression to calculate the rate of heat rejected from the system
Here, specific enthalpy at the exit of high pressure compressor is
Write the general expression to calculate the exergy destruction
Here, mass flow rate is
Conclusion:
From the Table A-11 of “Saturated refrigerant R-134a: Temperature”, obtain the properties of refrigerant at high pressure compressor inlet temperature
From the Table A-13 of “Superheated refrigerant R-134a”, obtain the specific enthalpy of high pressure compression exit at pressure
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at high pressure cycle throttle inlet pressure of
Substitute
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at high pressure cycle throttle exit temperature of
Substitute
Substitute
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at low pressure cycle throttle inlet temperature of
Here, pressure of refrigerant at the separator is
Substitute
From the Table A-11 of “Saturated refrigerant R-134a: Temperature”, obtain the properties of refrigerant at low pressure expansion valve exit temperature
Substitute
Substitute 0.2437 for
From the Table A-11 of “Saturated refrigerant R-134a: Temperature”, obtain the properties of refrigerant at low pressure compressor inlet temperature
From the Table A-13 of “Superheated refrigerant R-134a”, obtain the specific enthalpy of low pressure compression exit at pressure
Substitute
Substitute
Substitute
Rewrite the Equation (VII) for the process 2 to 3.
Here, the temperature of the high temperature reservoir is
Substitute
Rewrite the Equation (VII) for the process 3 to 4.
Substitute
Rewrite the Equation (VII) for the process 5 to 6.
Substitute
Rewrite the Equation (VII) for the process 6 to 7.
Substitute
Rewrite the Equation (VII) for the separator process.
Substitute
The exergy destruction for the isentropic processes is zero. Hence,
Thus, the greatest amount of exergy destroyed among the processes of system is
Want to see more full solutions like this?
Chapter 11 Solutions
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





