
Repeat Prob. 11–56 for a flash chamber pressure of 0.6 MPa.
(a)
The fraction of the refrigerant that evaporates as it is throttled to the flash chamber.
Answer to Problem 57P
The fraction of the refrigerant that evaporates as it is throttled to the flash chamber is
Explanation of Solution
Show the T-s diagram for compression refrigeration cycle as in Figure (1).
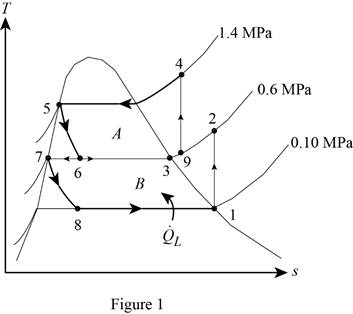
From Figure (1), write the specific enthalpy at state 5 is equal to state 6 due to throttling process.
Here, specific enthalpy at state 5 and 6 is
From Figure (1), write the specific enthalpy at state 7 is equal to state 8 due to throttling process.
Here, specific enthalpy at state 7 and 8 is
Express the fraction of the refrigerant that evaporates as it is throttled to the flash chamber
Here, specific enthalpy at saturated vapor is
Conclusion:
Perform unit conversion of pressure at state 1 from
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 1
Here, specific entropy and enthalpy at state 1 is
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2 corresponding to pressure at state 2 of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy at state 2 and specific enthalpy at state 2 respectively.
Show the specific enthalpy at state 2 corresponding to specific entropy as in Table (1).
|
Specific entropy at state 2 |
Specific enthalpy at state 2 |
| 0.9500 | 270.83 |
| 0.9519 | |
| 0.9817 | 280.60 |
Substitute
Thus, the specific enthalpy at state 2 is,
Perform unit conversion of pressure at state 3 from
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 3
Perform unit conversion of pressure at state 5 from
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 5
Here, specific enthalpy at saturated liquid is
Substitute
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 8
Substitute
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the specific enthalpy at evaporation and pressure of
Substitute
Hence, the fraction of the refrigerant that evaporates as it is throttled to the flash chamber is
(b)
The rate of heat removed from the refrigerated space.
Answer to Problem 57P
The rate of heat removed from the refrigerated space is
Explanation of Solution
Express the enthalpy at state 9 by using an energy balance on the mixing chamber.
Here, the rate of total energy entering the system is
Express the mass flow rate through the flash chamber.
Here, mass flow rate through condenser is
Express The rate of heat removed from the refrigerated space.
Conclusion:
Substitute
Substitute
Substitute
Hence, the rate of heat removed from the refrigerated space is
(c)
The coefficient of performance.
Answer to Problem 57P
The coefficient of performance is
Explanation of Solution
Express compressor work input per unit mass.
Express the coefficient of performance.
Express entropy at state 4.
Here, specific entropy at state 3 is
Conclusion:
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the property corresponding to pressure at state 3
Here, specific entropy at saturated vapor is
Substitute
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 4 corresponding to pressure at state 4 of
Show the specific enthalpy at state 4 corresponding to specific entropy as in Table (2).
|
Specific entropy at state 4 |
Specific enthalpy at state 4 |
| 0.9389 | 285.47 |
| 0.9444 | |
| 0.9733 | 297.10 |
Use excels and substitute value from Table (2) in Equation (IV) to get,
Substitute
Substitute
Hence, the coefficient of performance is
Want to see more full solutions like this?
Chapter 11 Solutions
Thermodynamics: An Engineering Approach
- what is the mechanism of natural gas Compressors?arrow_forwardAs a car gets older, will its compression ratio change? How about the mean effective pressure?arrow_forwardExplain the thermodynamic processes involved in a multi-stage compression system, outlining the specific efficiency considerations at each stage, and how these processes differ from a single-stage compression system.arrow_forward
- Does the efficient shaft have a circular cross-section?arrow_forward21 - The mass flow rate of water entering a turbine is 17.7 kg/s and its enthalpy is 3817.2 kJ/kg. The water exits the turbine as wet steam at a pressure of 100 kPa with a dryness degree of 0.92. What is the power produced in the turbine? a) 23413.4 kW B) 15476.6 kW NS) 13951.5 kW D) 11802.9 kW TO) 20271.5 kWarrow_forward16-Water vapor enters an isentropic turbine at a pressure of 3 MPa and a temperature of 400°C and exits the turbine at a pressure of 100 kPa. What is the degree of dryness at the turbine outlet? A) % 92,8 B) % 81,5 C) % 76,4 D) % 95,5 E) % 89,9arrow_forward
- a petrol working on a constant volume cycle has a compression ratio of 9 to 1. if the pressure and temperature of the petrol-vapor mixture at the beginning of compression assuming it follows the law PV^1.4=Carrow_forwardHow did the ideal gas laws apply in internal combustion engines?arrow_forwardHow do you calculate the heating surface of a boiler?arrow_forward
- Show that the air standard efficiency of a gas turbine can be expressed in terms ofpressure ratio only.arrow_forwardHow do the different zones of gas turbine combustor affect the formation of pollutants?arrow_forwardDetermine the temperatures and pressures at all stages of an ideal air-standard SI Otto cycle analysisarrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
