
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
1st Edition
ISBN: 9781111580704
Author: Kevin D. Dahm, Donald P. Visco
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.12, Problem 23P
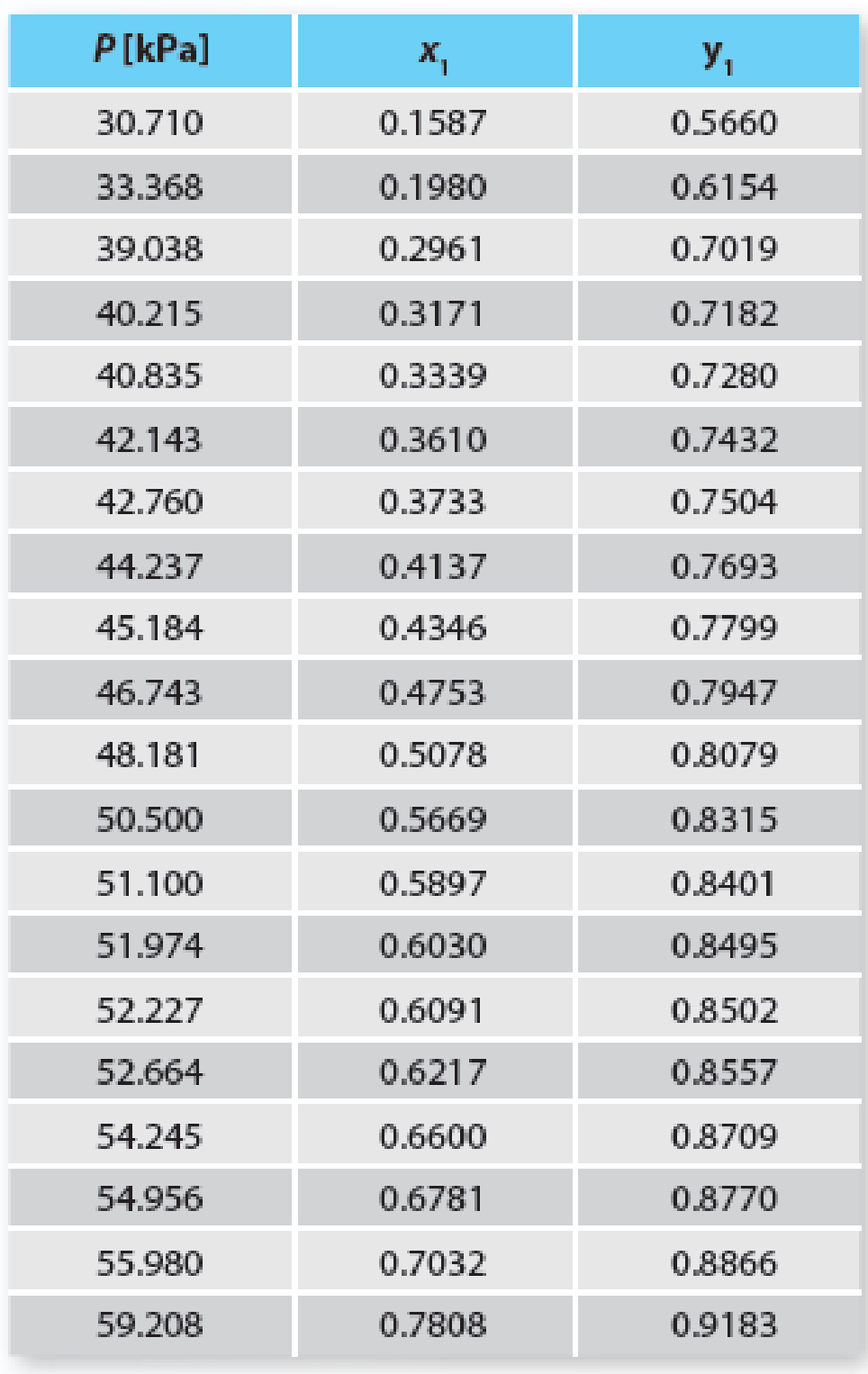
You are interested in evaluating how well you can predict the phase-behavior of a system using an excess molar Gibbs free energy model. Here, use the van Laar predictions (from the VDW-defined parameters) relative to fitting the data (in Table P11-23) with the van Laar model for the methanol (1) + water (2) system at 328.15 K. What is your conclusion based on these results?
TABLE P11-23 Vapor-liquid equilibrium of methanol (1) + water (2) at 328.15 K.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
Ch. 11.11 - The derivation of the expression for the natural...Ch. 11.11 - Derive the expression for the natural logarithm of...Ch. 11.11 - Prob. 3ECh. 11.11 - Given an equimolar binary mixture, calculate the...Ch. 11.11 - Prob. 5ECh. 11.11 - Prob. 6ECh. 11.11 - Calculate the van Laar parameters, L12 and L21,...Ch. 11.11 - Calculate the van Laar parameters using the...Ch. 11.11 - Explain why the integral test for thermodynamic...Ch. 11.11 - Prob. 10E
Ch. 11.12 - A binary liquid containing mostly component 2 is...Ch. 11.12 - Provide an estimate of the composition of N2...Ch. 11.12 - Resolve Example Problem 11.1, but now include the...Ch. 11.12 - A liquid mixture of 20% by mole benzene and the...Ch. 11.12 - You are interested in finding the pressure at...Ch. 11.12 - For a chloroform (1) + ethanol (2) system at 55C,...Ch. 11.12 - You are interested in finding the pressure at...Ch. 11.12 - The azeotrope of a binary mixture, being an...Ch. 11.12 - Consider the experimental data in Table P11-21 for...Ch. 11.12 - In the sizing of separation equipment, you need to...Ch. 11.12 - You are interested in evaluating how well you can...Ch. 11.12 - Compare the van Laar predictions if using...Ch. 11.12 - You desire to flash 20 moles/min of a liquid...Ch. 11.12 - Your company needs to evaluate the separation of...Ch. 11.12 - In a process you need to evaluate the flash...Ch. 11.12 - You desire to flash separate 10 mol/s of an...Ch. 11.12 - In a process analysis application, you are working...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Homogeneous and Heterogeneous Equilibrium - Chemical Equilibrium - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=8V9ozZSKl9E;License: Standard YouTube License, CC-BY