
ORGANIC CHEMISTRY
8th Edition
ISBN: 9781323815427
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.4, Problem 21P
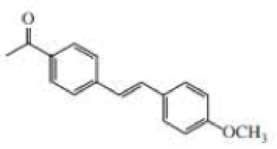
Identify two pairs of an alkyl bromide and an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Rank the compounds according to their increasing reactivity toward electrophilic substitution.
1. Benzaldehyde
2. o-dimethylbenzene
3. nitrobenzene
4. phenol
name the major product for the reaction of 4-methyl-1-pentyne with the following reagents
1 mol HBr
2 mol HBr
1 mol Br2/CH2Cl2
What product is obtained from the reaction of excess benzene with each of the following reagents?
a. isobutyl chloride + AlCl3
b. 1-chloro-2,2-dimethylpropane + AlCl3
c. dichloromethane + AlCl3
Chapter 11 Solutions
ORGANIC CHEMISTRY
Ch. 11.1 - Prob. 1PCh. 11.2 - Which is more reactive an organolithium compound...Ch. 11.2 - Prob. 3PCh. 11.3 - Muscalure is the sex attractant of the common...Ch. 11.3 - Prob. 7PCh. 11.3 - Prob. 8PCh. 11.3 - Prob. 9PCh. 11.3 - Prob. 10PCh. 11.4 - Prob. 13PCh. 11.4 - Prob. 14P
Ch. 11.4 - Prob. 15PCh. 11.4 - Prob. 16PCh. 11.4 - Prob. 17PCh. 11.4 - Prob. 19PCh. 11.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 11.4 - Identify two pairs of an alkyl bromide and an...Ch. 11.5 - Prob. 22PCh. 11.5 - Draw the product of ring-closing metathesis for...Ch. 11.5 - Prob. 25PCh. 11.5 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - The coupling of an alkyne with an aryl halide in...Ch. 11 - Identify A through H.Ch. 11 - Using the given starting material, any necessary...Ch. 11 - What alkyl halide reacts with lithium...Ch. 11 - Prob. 33PCh. 11 - Prob. 34PCh. 11 - The following compound undergoes an intramolecular...Ch. 11 - Using ethynyleyclohexane as a starting material...Ch. 11 - Prob. 37PCh. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 39PCh. 11 - A student added an equivalent of...Ch. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 42PCh. 11 - Prob. 43PCh. 11 - Bombykol is the sex pheromone of the silk moth....Ch. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - A dibromide loses only one bromine when it reacts...Ch. 11 - What starting material is required in order to...Ch. 11 - What product is obtained from ring-opening...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a deuterated compound?arrow_forward) Which of the following scientific statements are true and which are false? 1- [ ] E1 describes an elimination reaction in which the rate-determining step does not involve the base. 2- [ ] Rate of formation of carbocations follow the order 3˚ > 2˚> 1˚> CH3+ 3- [ ] Cyclohexanol is oxidized to ketone using Jone's reagent. 4- [ ] Symmetric ethers are generally synthesized by dehydration of alcohols. 5- [ ] The SN2 reaction occurs with inversion of configuration.arrow_forwardWhich of the following compounds would react most vigorously with liquid bromine when no light is present? A) cyclohexane B) Hexane C) benzene D) hex-2-enearrow_forward
- Choose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) Br2, FeBr3 Na/NH3, -33 degrees C NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heatarrow_forward1. Predict the elimination products of the following reactions. When two alkenes are possible, predict which one will be the major product. Explain your answers, showing the degree of substitution of each double bond in the products. 2. Which of these reactions are likely to produce both elimination and substitution products? (a) 2-bromopentane +NaOCH3 (b) 3-bromo-3-methylpentane +NaOMe(Me= methyl, CH3) (c) 2-bromo-3-ethylpentane +NaOH (d) cis-1-bromo-2-methylcyclohexane +NaOEt (Et= ethyl, CH2CH3)arrow_forwardWhat is the expected major product of reacting cyclohexane carbaldehyde with: 1) CH3MgBr 2) H3O+arrow_forward
- 1. Which reaction conditions would be best for turning ethene into ethanol? a.H2O, HBr b. CH3OH, H2SO4 c.CH3OH, HBR d. H2O, H2SO4 2. Which alkene would be non-regioselective in a reaction with HBr? a. 2-ehyl-2-methyl-2-butene b.2-ethyl-3-methyl-2-butene c. 2,3-dimethyl-2-butene d.1,2-dimethyl-2-butenearrow_forwardDraw the four resonance structures formed during bromination of methoxybenzene, CH3OC6H5, with Br2/FeBr3 to give para-bromomethoxybenzene and then circle the most stable resonance form.arrow_forwardRank the compounds in each group according to their reactivity toward electrophilic substitution: (a) Chlorobenzene, o-dichlorobenzene, benzene (b) p-Bromonitrobenzene, nitrobenzene, phenol (c) Fluorobenzene, benzaldehyde, o-dimethylbenzenearrow_forward
- Predict the products of reaction of pent-1-yne with the following reagents. Na, liquid ammoniaarrow_forwardChoose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) KMnO4, H3O+ CH3Cl, AlCl3 HNO3, H2SO4 Cl2, FeCl3 fuming sulfuric acidarrow_forwardAnother important pattern in organic synthesis is the construction of CC bonds. Using your reaction roadmap as a guide, show how to convert propane into hex-1-en-4-yne. You must use propane as the source of all of the carbon atoms in the hex-1-en-4-yne product. Show all reagents needed and all molecules synthesized along the way.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY