
Concept explainers
(a)
Interpretation:
The following ball and stick model should be converted to skeletal or condensed formula and the
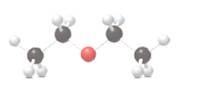
Concept Introduction:
Ball and stick model of molecule is determined by X-ray crystallography, in which the hydrogen atoms are depicted as white balls, carbon atoms as black balls and oxygen atom as red balls.
Organic molecules have some structural features in addition to
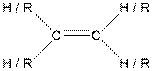
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
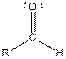 | |
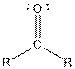 | |
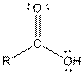 | |
| Ester | 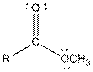 |
| Amide | 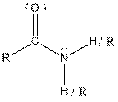 |
| Alcohol |  |
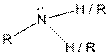 | |
 | |
| Ether | 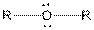 |
Here, R = any carbon backbone
(b)
Interpretation:
The following ball and stick model should be converted to skeletal or condensed formula and the functional groups present in the compound should be recognized:
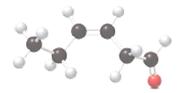
Concept Introduction:
Ball and stick model of molecule is determined by X-ray crystallography, in which the hydrogen atoms are depicted as white balls, carbon atoms as black balls and oxygen atom as red balls.
Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 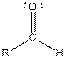 |
| Ketone | 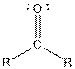 |
| Carboxylic acid | 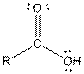 |
| Ester | 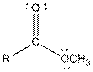 |
| Amide | 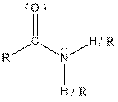 |
| Alcohol |  |
| Amine | 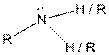 |
| Alkene | 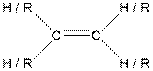 |
Here, R = any carbon backbone
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- How many electron pairs are shared when a triple bond exists between two carbon atoms? What must he the geometric arrangement around the carbon atoms in a triple bond? Draw the Lewis structure of a simple molecule that contains a triple bond.arrow_forwardWrite condensed structural formulas, and skeletal structures for each structural isomer of C4H10.arrow_forward= NAMING AND DRAWING ORGANIC MOLECULES Drawing a skeletal structure from a condensed structure Draw a skeletal ("line") structure of this molecule: CH3 OH -6-6- CH3-CH₂-C=C-CH3 Click and drag to start drawing a structure.arrow_forward
- Write the condensed structure for each of these skeletal structures. Cl condensed structure: condensed structure:arrow_forwardDraw the condensed structure of an isomer of this molecule: OH CH₂ C-CH₂-OHarrow_forwardDraw the condensed structure for Butane and please follow the steps below to answer the question. Step 1: Draw the 2-dimensional structural formula for the molecule Step 2: Identify the carbon backbone (or skeleton) and any branches or side-chains in the structural formula as well as any functional groups such as halogen (group 17) atoms. Step 3: Identify groups of atoms along the carbon backbone in the 2-dimensional structural formula. Step 4: Re-draw the formula by replacing the identified groups of atoms in the 2-dimensional structural formula with their condensed structure, or semi-structural formula. Step 5: Remove single carbon-carbon covalent bonds from the backbone or skeleton (this step is not absolutely necessary). Step 6: If the molecule contains branches or side-chains: (a) condense the structure as for the carbon backbone (b) use parantheses (round brackets) to enclose groups of atoms attached to the chain (c) use a subscript number to indicate the number of times this…arrow_forward
- Draw the condensed structure for Butane and please follow the steps below to answer the question. Step 1: Draw the 2-dimensional structural formula for the molecule Step 2: Identify the carbon backbone (or skeleton) and any branches or side-chains in the structural formula as well as any functional groups such as halogen (group 17) atoms. Step 3: Identify groups of atoms along the carbon backbone in the 2-dimensional structural formula. Step 4: Re-draw the formula by replacing the identified groups of atoms in the 2-dimensional structural formula with their condensed structure, or semi-structural formula. Step 5: Remove single carbon-carbon covalent bonds from the backbone or skeleton (this step is not absolutely necessary). Step 6: If the molecule contains branches or side-chains: (a) condense the structure as for the carbon backbone (b) use parantheses (round brackets) to enclose groups of atoms attached to the chain (c) use a subscript number to indicate the number of times this…arrow_forwardDetermine the molecular formulas and then write line-angle (skeletal) structures for the following condensed structures. Do these structures represent the same molecule? explain. CH3CH2COCH2C(CH3)3 CH3COCH2C(CH3)3 CH3CH(OH)CH2CH2C(CH3)3arrow_forward4: Draw out the abbreviated structural formula of the following molecules. 4-Methyl-2-hexene 2-Methyl-2-hexene 2-Hexene 5: Draw the structure of 2-Butene, labeling the n bond and all the B bonds. 6: Draw all the structures possible with the formula CSH8.arrow_forward
- I have given you a condensed structure. You need to convert it to an accurate bond-line structure. CH3 CH3-CH-CH2-CH-CH₂-C-H 12-CH-CH2₂-C-1 CI Draw (as bond-line structures) isomers of this compound where you only move the chlorine atom. Draw four isomers of the original compound that would have a five carbon chain as the longest chain. [Note: there would be many isomers that will satisfy this. Find any four.] Using the original molecule (and looking at the carbon next to the aldehyde carbonyl) what would be the charge on that carbon if I removed an H atom and left behind the pair of electrons? Circle the best answer Positive Negative Neutral In the space below, draw that structure (from the sentence above) as a bond-line structure. Then, draw a resonance structure for this ion and be sure to add curved arrows to show the movement of electrons.arrow_forwardBased on the structures below: i) Convert the structures above to the skeletal structure.ii) Identify the functional groups and homologous series in each compound.arrow_forwardConvert the following molecular model into a condensed structure and a skeletal structure.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

