
(a)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(b)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(c)
Interpretation:
Whether the cyclohexane ring “breathing” or simultaneous stretching of the all
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The simultaneous stretch of all
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The simultaneous stretch of all
Therefore, simultaneous stretch of all
The simultaneous stretch of all
(d)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(e)
Interpretation:
Whether the symmetrical stretching of the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The symmetric stretch of
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The symmetric stretch of
Therefore, symmetric stretch of
The symmetric stretch of
(f)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The stretch of
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The stretch of
Therefore, stretch of
The stretch of
(g)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The structure of
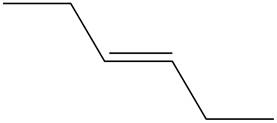
Figure 1
The
Therefore,
The
Want to see more full solutions like this?
Chapter 12 Solutions
Loose-leaf Version For Organic Chemistry
- What is the frequency range of the characteristic absorption bands of the following bonds? (a) C=O _______________ (b) O-H _______________ (c) C=C _______________ (d) C-C _______________arrow_forwardIf chloroform (trichloromethane) exhibits an infrared peak at 3018cm−1 due to C–H stretching vibration, calculate wavenumber of the absorption band corresponding to C–D stretching vibration in deuterochloroform (experimental value 2253cm−1).arrow_forwardConvert the following wavenumbers (in cm-1) into their corresponding wavelengths (in nm) a. 3300 cm-1 b. 3050 cm-1 c. 2950 cm-1 d. 1700 cm-1 e. 750 cm-1arrow_forward
- C2H5ClO2 elucidate thefollowing spectrum and identify the unknown compoundarrow_forwardQ.2. (a) An atomic absorption spectrometer requires a very different light source than does aninstrument for molecular absorption. Describe the light source used by each instrumentand explain why these two instruments need such different light sources. As part of youranswer, explain the consequences if you interchange the two types of sources. (b) IR Spectroscopy has various sources for producing IR spectrum but we cannot use thesein X-ray and Mass Spectroscopy, give explanation.arrow_forwardRotational absorption lines from 1H35Cl gas were found at the following wavenumbers (R.L. Hausler and R.A. Oetjen, J. Chem. Phys. 21, 1340 (1953)): 83.32, 104.13, 124.73, 145.37, 165.89, 186.23, 206.60, 226.86 cm−1. Calculate the moment of inertia and the bond length of the molecule. Predict the positions of the corresponding lines in 2H35Cl.arrow_forward
- Calculate the absorption energy observed at 8.5 x 10 ^ -3 cm ^ -1 (h = 6.62 x 10 ^ -34 J.s, c = 3 x 10 ^ 10 cm / s) E = hu; c = lu. Pleas Show your work in detail if h = 6.62 x 10 ^ -34 J.s and c = 3.0 x 10 ^ 10 cm / sec.arrow_forwardWhat is the Nuclear Magnetic Resonance spectrum of CH3-CH(OCH2CH3)2arrow_forwardCalculate the energy of the absorption observed at 9.56 x10 ^ -3 cm (h 6.62 x10 ^ -34 J.s, c = 3x10 ^ 10 cm / s).arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

