
Concept explainers
a.
Interpretation:
The time to heat the sample to its melting point is to be calculated.
Concept introduction:
Specific heat capacity
Here,
The formula to calculate heat at phase change is as follows:
Here,
a.
Answer to Problem 12.142P
The time to heat the sample to its melting point is
Explanation of Solution
Substitute
Constant rate of heating is
The given sample reaches its melting point by heating for
(b)
Interpretation:
The time taken to melt the sample is to be calculated.
Concept introduction:
Specific heat capacity
Here,
The formula to calculate heat at phase change is as follows:
Here,
(b)
Answer to Problem 12.142P
The time taken to melt the sample is
Explanation of Solution
Substitute
Constant rate of heating is
The time taken to melt
(c)
Interpretation:
A curve of temperature vs. time for the entire heating process is to be calculated.
Concept introduction:
Specific heat capacity
Here,
The formula to calculate heat at phase change is as follows:
Here,
(c)
Answer to Problem 12.142P
The curve of temperature vs. time for the entire heating process is as follows:
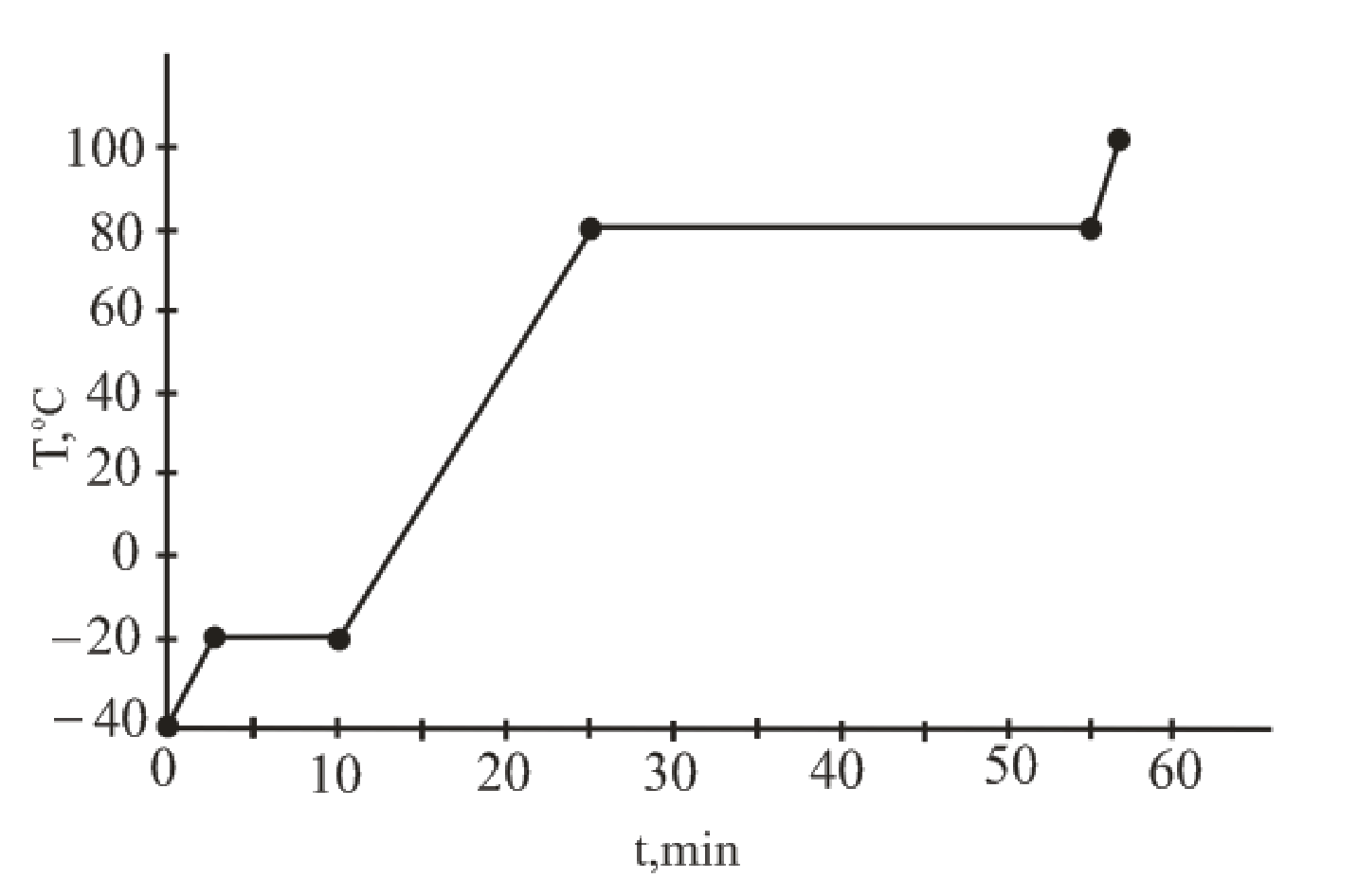
Explanation of Solution
Substitute
Constant rate of heating is
Substitute
Constant rate of heating is
Substitute
Constant rate of heating is
The curve of temperature vs. time for the entire heating process is as follows:
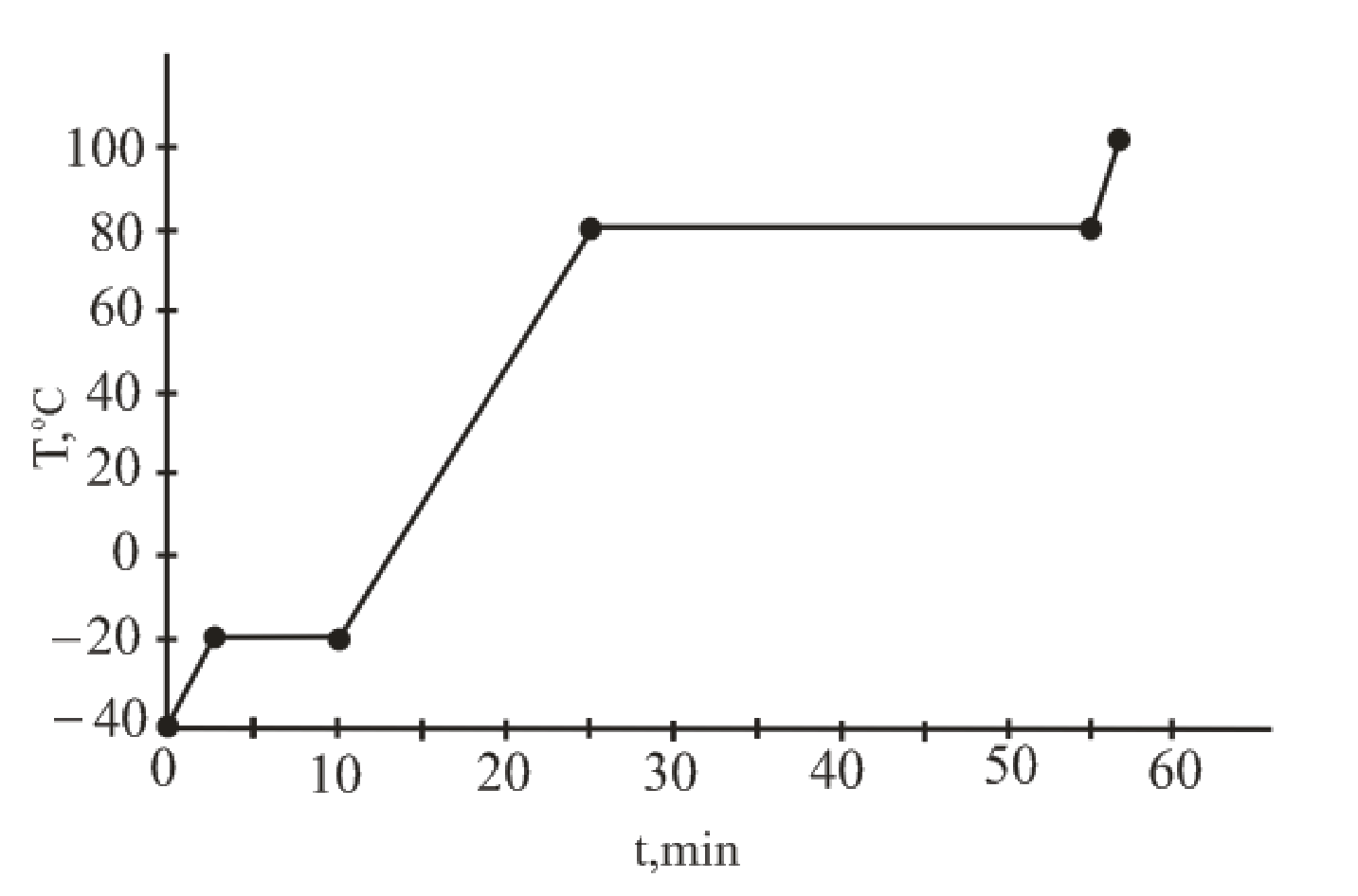
The temperature versus time plot for the given sample represents the phase change.
Want to see more full solutions like this?
Chapter 12 Solutions
GEN CMB CHEM; CNCT+;ALEKS 360
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





