
(a)
Interpretation:
The complete, detailed mechanism and the major organic product for the given reaction are to be drawn.
Concept introduction:
When an
Answer to Problem 12.37P
The complete, detailed mechanism and the major organic product for the given reaction are shown below:
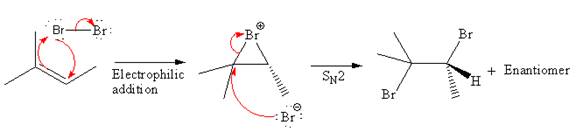
Explanation of Solution
The starting material for the given reaction is

When alkene is treated with molecular bromine
In step 2, the
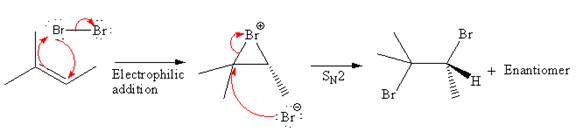
The complete, detailed mechanism and the major organic product for the given reaction is drawn by electrophilic addition of
(b)
Interpretation:
The complete, detailed mechanism and the major organic product for the given reaction is to be drawn.
Concept introduction:
Molecular halogen undergoes anti addition across a
Answer to Problem 12.37P
The complete, detailed mechanism and the major product for the given reaction is as shown below:
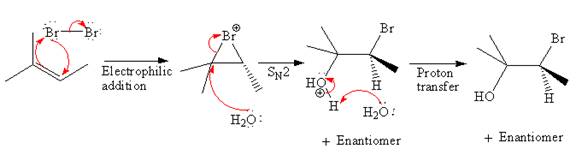
Explanation of Solution
The starting material for given reaction is

In the first step,
The complete, detailed mechanism and the major product for the given reaction is as shown below:
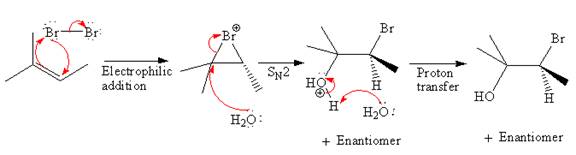
The detailed mechanism for the given reaction is described by explaining the appropriate stereochemistry with the formation of bromonium ion intermediate.
(c)
Interpretation:
The complete, detailed mechanism and the major organic product for the given reaction is to be drawn.
Concept introduction:
When an alkene reacts with molecular chlorine
Answer to Problem 12.37P
The complete, detailed mechanism and the major organic product for the given reaction are shown below:
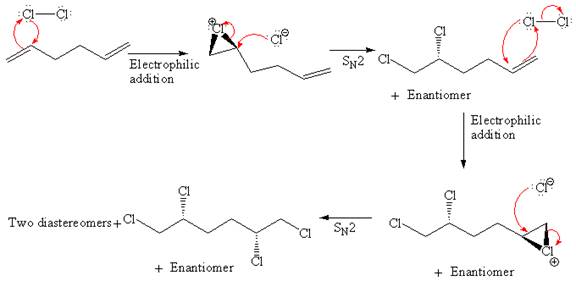
Explanation of Solution
The starting material for given reaction is
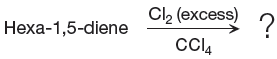
When alkene is treated with molecular chlorine in
In step 2, the
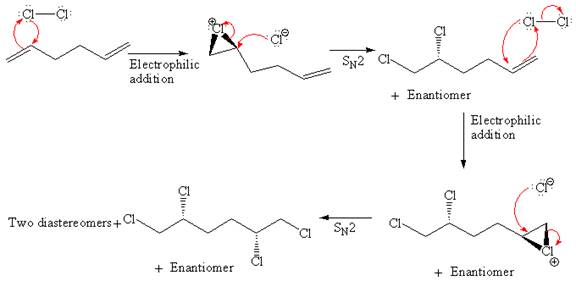
The complete, detailed mechanism and the major organic product for the given reaction are drawn by electrophilic addition of
(d)
Interpretation:
The complete, detailed mechanism and the major organic product for the given reaction is to be drawn.
Concept introduction:
When an
Answer to Problem 12.37P
The complete, detailed mechanism and the major organic product for the given reaction are shown below:
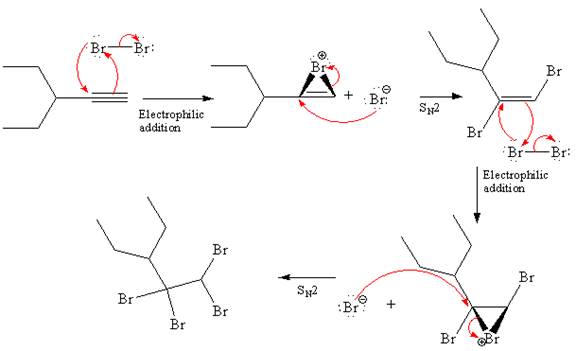
Explanation of Solution
The starting material for the given reaction is
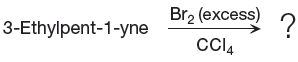
When alkyne is treated with molecular bromine
In step 2, the

The complete, detailed mechanism and the major organic product for the given reaction are drawn by electrophilic addition of
Want to see more full solutions like this?
Chapter 12 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Draw the complete, detailed mechanism for the reaction shown here and give the major product. CH3I (excess) ? NH2arrow_forwardOne way to synthesize diethyl ether is to heat ethanol in the presence of a strong acid, as shown here. Draw a complete, detailed mechanism for this reaction.arrow_forwardDraw the complete, detailed mechanism for the following reaction.arrow_forward
- Draw the complete, detailed mechanism for the reaction shown here. Will the product be optically active? Explain.arrow_forwardDraw the complete, detailed mechanism for the following reaction along with the major product. Cl2 HOẠCarrow_forwardPlease draw out the whole mechanism for the following reaction.arrow_forward
- For each reaction, draw the complete, detailed mechanism and the major product. NaCl HCI ?arrow_forwardDraw the detailed mechanism and the product for the following reaction. Be sure to include stereochemistry, if applicable. Brarrow_forwardThe reaction shown here is an example of the Favorskii reaction, which involves an R¯ leaving group in a nucleophilic addition-elimination reaction. (a) Draw the complete, detailed mechanism for this reaction and explain why R can act as a leaving NaOH H2O group. (b) Suggest how you can synthesize an ester from cyclopropanone using only this reaction.arrow_forward
- Provide a complete, detailed mechanism for the reaction CH;CH,OH shown here.arrow_forwardDraw a complete, detailed mechanism for the reaction CH3CH2OH shown here. Harrow_forwardDraw the complete, detailed mechanism for the reaction shown here and draw the major organic product. NH3 C,H15N H2O, Aarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





