
(a)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
(a)
Explanation of Solution
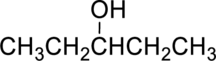
The given alcohol is,
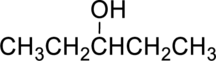
Parent compound : pentane
Position of –OH : carbon – 3
Substituent : No substituent present
IUPAC Name : pentan-3-ol
Therefore, the
Pentan-3-ol is a secondary alcohol it undergoes oxidation reaction to produce 3-pentanone. The reaction can be shown as follows,
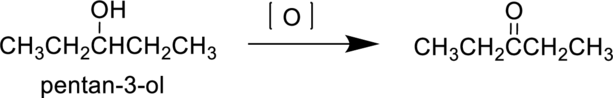
Parent compound : pentane
Position of
Substituent : No substituent present
IUPAC Name : pentan-3-one
Therefore, the IUPAC nomenclature for the given product is pentan-3-one.
(b)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
(b)
Explanation of Solution
The given alcohol is,
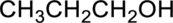
Parent compound : propane
Position of –OH : carbon – 1
Substituent : No substituent present
IUPAC Name : propan-1-ol
Therefore, the IUPAC nomenclature for the given alcohol is propan-1-ol.
Propan-1-ol is a primary alcohol it undergoes oxidation reaction to produce propanal and forms propanoic acid on further oxidation. The reaction can be shown as follows,
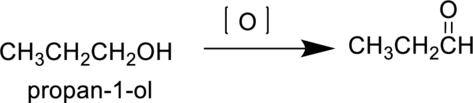
Parent compound : propane
Position of
Substituent : No substituent present
IUPAC Name : propan-1-al
Therefore, the IUPAC nomenclature for the given product is propan-1-al.
(c)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
(c)
Explanation of Solution
The given alcohol is,
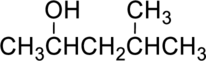
Parent compound : pentane
Position of –OH : carbon – 2
Substituent : 4-methyl
IUPAC Name : 4-methylpentan-2-ol
Therefore, the IUPAC nomenclature for the given alcohol is 4-methylpentan-2-ol.
4-methylpentan-2-ol is a secondary alcohol it undergoes oxidation reaction to produce 4-methylpentan-2-one. The reaction can be shown as follows,
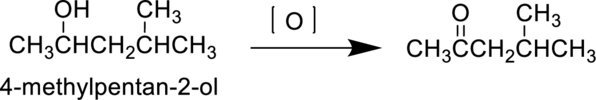
Parent compound : pentane
Position of
Substituent : 4-methyl
IUPAC Name : 4-methylpentan-2-one
Therefore, the IUPAC nomenclature for the given product is 4-methylpentan-2-one.
(d)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
(d)
Explanation of Solution
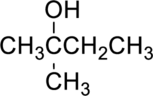
The given alcohol is,
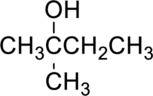
Parent compound : butane
Position of –OH : carbon – 2
Substituent : 2-methyl
IUPAC Name : 2-methylbutan-2-ol
Therefore, the IUPAC nomenclature for the given alcohol is 2-methylbutan-2-ol.
2-methylbutan-2-ol is a tertiary alcohol it does not undergoes oxidation reaction. Hence the it considered as N.R and it can be shown as follows,
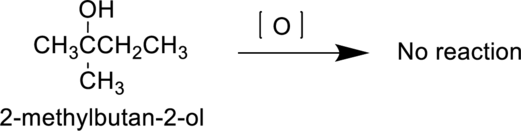
(e)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
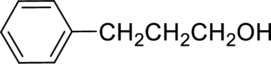
(e)
Explanation of Solution
The given alcohol is,
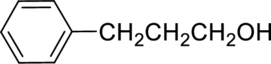
Parent compound : propane
Position of –OH : carbon – 1
Substituent : 3-phenyl
IUPAC Name : 3-phenylpropan-1-ol
Therefore, the IUPAC nomenclature for the given alcohol is 3-phenylpropan-1-ol.
3-phenylpropan-1-ol is a primary alcohol it undergoes oxidation reaction to produce 3-phenylpropan-1-al and forms 3-phenylpropanoic acid on further oxidation. The reaction can be shown as follows,
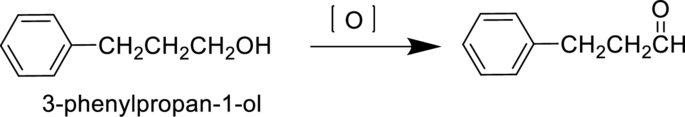
Parent compound : propane
Position of
Substituent : 3-phenyl
IUPAC Name : 3-phenylpropan-1-al
Therefore, the IUPAC nomenclature for the given product is 3-phenylpropan-1-al.
Want to see more full solutions like this?
Chapter 12 Solutions
General, Organic, And Biochemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





