
Concept explainers
Interpretation:
The limiting reactant in the reaction of
Concept Introduction:
The reactant that determines the concentration of the product formed in a chemical reaction is known as limiting reagent. The other reactants left in the reaction mixture are termed as reactants in excess.
Answer to Problem 12.71E
The limiting reactant in the reaction of
Explanation of Solution
The reaction of butane with iodine results in the formation of
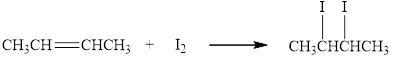
Figure 1
The molecular formula of
The molar mass of
The molar mass of
The number of moles is calculated by the formula shown below.
Substitute molar mass of and given mass of
Therefore, the number of moles of
The molar mass of iodine is
The molar mass of
The molar mass of
Substitute molar mass of and given mass of
Therefore, the number of moles of
The number of moles of
The limiting reactant in the reaction of
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Heptane, C7H16, can be catalytically reformed to make toluene, C6H5CH3, another seven-carbon molecule. How many hydrogen molecules are produced for every toluene molecule derived from heptane? Write a balanced chemical equation for this reaction. Why is it profitable to convert heptane into toluene?arrow_forward4.61 What is actually measured by the octane ratings of different grades of gasoline?arrow_forwardIn the combustion of one molecule of 4-5diethyl-3-methyloctane,How many molecules of water are produced ?arrow_forward
- In the complete combustion of octane the balanced reaction has one mole of the hydrocarbon. How many moles of oxygen are present?arrow_forwardThey are all part of one question. Ethanol, C2H6O, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The heat of combustion of ethanol is 326.7 kcal/mol.The heat of combustion of 2-methylhexane, C7H16, is 1.150×103 kcal/mol. How much energy is released during the complete combustion of 495 grams of 2-methylhexane ? ______kcalAssuming the same efficiency, would 495 grams of ethanol provide more, less, or the same amount of energy as 495 grams of 2-methylhexane? Hydrocarbons, compounds containing only carbon and hydrogen, are important in fuels.The heat of combustion of cyclohexane, C6H12, is 936.8 kcal/mol.Write a balanced equation for the complete combustion of cyclohexane. + + How much energy is released during the complete combustion of 364 grams of cyclohexane ? _______kcal Combustion…arrow_forwardThe complete combustion of one mole of 2,3-dimethyl-4-(1-methylethyl)heptane produces how many moles of H2O?arrow_forward
- How many molecules of carbon dioxide are produced from the combustion of 1.00g of glucose (C6H12O6)?arrow_forwardYields in organic reactions are sometimes low. What is the percent yield of a process that produces 13.0 g of ethyl acetate from 10.0 g of CH3CO2H?arrow_forwardThe combustion of methane is represented by the equation:CH4 + 2O2 → CO2 + 2H2Oa) In the above reaction what compound is oxidized?b) Give another example of a hydrocarbon combustion reaction and write the equation.arrow_forward
- The complete combustion of two moles of 2-methylpropane would yield how many moles of carbon dioxide (CO₂)? A) 2 B) 4 C) 8 D) 16arrow_forwarda) Calculate the heat evolve when the ethanol was combusted b) Calculate the enthalpy change of combustion per mole of ethanolarrow_forwardHow many grams of water are formed when 25.0 grams of butane are combusted in the presence of 60.0 grams of oxygen?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





