
Concept explainers
Lysozyme is an enzyme that cleaves bacterial cell walls. A sample of lysozyme extracted from egg white has a molar mass of 13,930 g. A quantity of 0.100 g of this enzyme is dissolved in 150 g of water at 25°C. Calculate the vapor-pressure lowering, the depression in freezing point, the elevation in boiling point, and the osmotic pressure of this solution. (The vapor pressure of water at 25°C is 23.76 mmHg.)
Interpretation:
For given solution vapor pressure lowering, freezing point depression, boiling point elevation and osmotic pressures to be calculated.
Concept introduction
Boiling point elevation
Where,
Freezing point depression
Where,
Osmotic pressure is the pressure that is needed to stop osmosis. Osmotic pressure of the solution is directly proportional to the concentration of the solution. We can calculate osmotic pressure by using this formula is given by,
Where,
Vapor pressure lowering: Vapor pressure lowering is one of the colligative properties. Pure solvent has higher vapour pressure than its solution have non-volatile liquid. Thus vapour pressure lowering guide boiling point elevation.
Where,
Answer to Problem 12.83QP
Vapour pressure lowering of the solution = 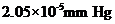
Freezing point elevation = 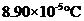
Boiling point elevation = 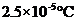
Osmotic pressure = 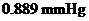
Explanation of Solution
Given data
Molar mass of egg white =
Amount of enzyme which is dissolved in water =
Amount of water =
Vapor pressure of water =
Calculation of number of moles in lysozyme and water
Molecular mass of water =
By plugging in the value of amount of Isozyme and molar mass of egg white, mole of Isozyme has calculated. Similarly, by plugging in the value of amount of water and molar mass of water, mole of water has calculated.
Calculation of vapour pressure lowering of the solution
By plugging in the values of mole fraction of Isozyme and vapour pressure of water, vapour pressure lowering of the solution has calculated.
Calculation freezing point depression of the solution
Molal freezing point depression constant =
By plugging in the values of molal freezing point depression constant and molality of the solution, freezing point depression of the solution has calculated.
Calculation of boiling point elevation of the solution
Boiling point elevation constant =
By plugging in the values of boiling point elevation constant and molality of the solution, boiling point elevation of the solution has calculated.
Calculation of osmotic pressure of the solution
As known above, we assume the density of the solution is
By plugging in the values of molarity of the solution, ideal gas constant and temperature in Kelvin, the osmotic pressure of the solution has calculated.
Vapour pressure lowering of the solution was calculated as
Freezing point elevation has calculated as
Boiling point elevation has calculated as
Osmotic pressure has calculated as
Want to see more full solutions like this?
Chapter 12 Solutions
CHEMISTRY-ALEK 360 ACCES 1 SEMESTER ONL
- What would be the freezing point of a solution formed by adding 1.0 mole of glucose (a molecular compound) to the following amounts of water? a. 250 g (0.25 kg) b. 500 g (0.500 kg) c. 1000 g (1.000 kg) d. 2000 g (2.000 kg)arrow_forwardCalculate the freezing point of 525 g of water that contains 25.0 g of NaCl. Assume i, the vant Hoff factor, is 1.85 for NaCl.arrow_forwardWater at 25 C has a density of 0.997 g/cm3. Calculate the molality and molarity of pure water at this temperature.arrow_forward
- Define the terms in Raoults law. Figure 10-9 illustrates the net transfer of water molecules from pure water to an aqueous solution of a nonvolatile solute. Explain why eventually all of the water from the beaker of pure water will transfer to the aqueous solution. If the experiment illustrated in Fig. 10-9 was performed using a volatile solute, what would happen? How do you calculate the total vapor pressure when both the solute and solvent are volatile?arrow_forwardConsider two solutions, A and B, separated by an osmotic semipermeable membrane that allows only water to pass through, as shown in the diagram in Problem 8-113. Based on each of the following identities for solutions A and B, indicate whether the liquid level in compartment A, with time, will increase, decrease, or not change. a. A = 1.0 M glucose solution and B = 2.0 M glucose solution b. A = 5.0%(m/v) NaCl solution and B = 4.0%(m/v) NaCl solution c. A = 2.0 M Na2SO4 solution and B = 3.0 M KNO3 solution d. A = 2.0 M glucose solution and B = 1.0 M NaCl solutionarrow_forwardErythrocytes are red blood cells containing hemoglobin. In a saline solution they shrivel when the salt concentration is high and swell when the salt concentration is low. In a 25C aqueous solution of NaCl, whose freezing point is 0.406C, erythrocytes neither swell nor shrink. If we want to calculate the osmotic pressure of the solution inside the erythrocytes under these conditions, what do we need to assume? Why? Estimate how good (or poor) of an assumption this is. Make this assumption and calculate the osmotic pressure of the solution inside the erythrocytes.arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





