
(a)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 33E
The electron dot formula for
![]()
The structural formula for
![]()
Explanation of Solution
The total number of valence electron in
![]()
Figure 1
The one electron pair is involved in bonding. Therefore, there is no lone pair. The structural formula for
![]()
Figure 2
The electron dot formula for
The structural formula for
(b)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 33E
The electron dot formula for
![]()
The structural formula for
![]()
Explanation of Solution
The total number of valence electron in
![]()
Figure 3
Out of seven electron pairs, only one electron pair is involved in bonding. Therefore, there is six lone pair. The structural formula for
![]()
Figure 4
The electron dot formula for
The structural formula for
(c)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 33E
The electron dot formula for
![]()
The structural formula for
![]()
Explanation of Solution
The total number of valence electron in
![]()
Figure 5
Out of four electron pairs, one electron pair is involved in bonding. Therefore, there is three lone pair. The structural formula for
![]()
Figure 6
The electron dot formula for
The structural formula for
(d)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 33E
The electron dot formula for
![]()
The structural formula for
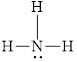
Explanation of Solution
The total number of valence electron in
![]()
Figure 7
Out of four electron pairs, three electron pair is involved in bonding. Therefore, there is one lone pair. The structural formula for
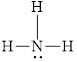
Figure 8
The electron dot formula for
The structural formula for
Want to see more full solutions like this?
Chapter 12 Solutions
INTRODUCTORY CHEMISTRY W/ACCESS
- Give a cation isoelectronic with fluoride ion______________________ 3.Arrange in increasingsize: sodiumion, nitride ion, magnesium ion, oxide ion, neon. (explain your rationale)______<______<______<_____<_____ Arrange in decreasingsize: sulfide ion, argon, potassium ion, calcium ion, chloride ion. (explain your rationale)______>______>______>_____>___arrow_forwardAmmonium nitrate is an inexpensive source of nitrogen content used to enrichfertilizers. Although when handled improperly, it could be dangerous andcatastrophic. What is the chemical formula for ammonium nitrate?arrow_forwardstate whether the following combinations of elements would be held together by ionic or covalent bondSodium and chlorinegallium and oxygennitrogen and hydrogenxenon and fluorineMagenesium and sulfursulfur and oxygenarrow_forward
- Use VSEPR theory to draw structures, with ideal bond an-gles, for boric acid and the anion it forms in reaction with water.arrow_forwardPredict the formula of a compound formed from:a. boron and hydrogenb. magnesium and phosphorusarrow_forwardIdentify the chemical formula of the molecule represented here:arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





