
(a)
Interpretation:
The synthetic route for the transformation of given molecules should be identified by using starting material with two carbon atoms.
Concept Introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
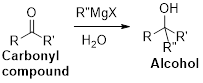
(b)
Interpretation:
The synthetic route for the transformation of given molecules should be identified by using starting material with two carbon atoms.
Concept Introduction:
Hydration:
When
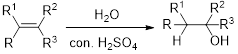
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (
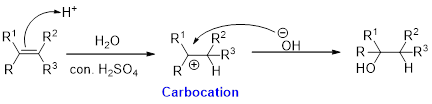
In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
(c)
Interpretation:
The synthetic route for the transformation of given molecules should be identified by using starting material with two carbon atoms.
Concept Introduction:
9-BBN (9-Borabicyclo[3.3.1]nonane) or diborane is used for the hydroboration of alkene. Boron addition to the double bond and subsequent oxidation of the new formed borane yields anti-Markovnikov alcohols.
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
(d)
Interpretation:
The synthetic route for the transformation of given molecules should be identified by using starting material with two carbon atoms.
Concept Introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
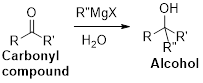
9-BBN (9-Borabicyclo[3.3.1]nonane) or diborane is used for the hydroboration of alkene. Boron addition to the double bond and subsequent oxidation of the new formed borane yields anti-Markovnikov alcohols.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEM.-PRINT COMP.(LL)W/2 ACCESS
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





