
Concept explainers
Methane is to be adiabatically and reversibly compressed from 50 psia and 100°F to 500 psia. Calculate the specific work required for this compression treating the methane as an ideal gas with variable specific heats and using the departure charts.
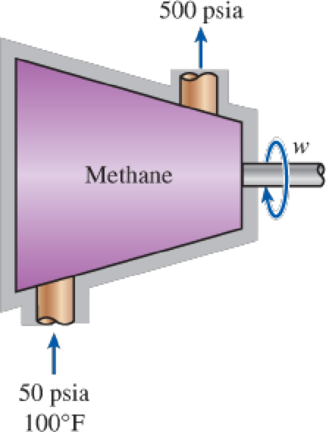
FIGURE P12–96E
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats and using departure charts.
Answer to Problem 96RP
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
The work input of the compressor per unit mass of the methane using departure charts is
Explanation of Solution
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
The general empirical correlation is
Write the formula for enthalpy change in molar basis at ideal gas state
Here, specific heat capacity at constant pressure is
Write the formula for work input to the compressor
Here, molar mass of methane is
Write the formula for entropy change in molar basis at ideal gas state
Here, universal gas constant is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Write the formula for change in enthalpy
Here, change in enthalpy of ideal gas is
Refer Table A-1E, “Molar mass, gas constant, and critical-point properties”.
The critical temperature and critical pressure of the methane is as follows.
Refer table A-1E, “Molar mass, gas constant and critical properties table”.
The molar mass
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
Obtain the empirical constants as follows.
Refer Table A-2E (a), “Ideal-gas specific heats of various common gases”.
The gas constant
The specific heat at constant pressure
The universal gas constant
Conclusion:
Convert the inlet temperature from degree Fahrenheit to Rankine.
It is given that the compression process in reversible adiabatic process. Hence, the change in entropy during the process is zero.
Substitute
By using Engineering Equation Solver (EES) or online calculator to solve the Equation (XI) and obtain the value of
Substitute
Substitute
Thus, the work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Consider the final temperature
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Substitute
Here, the work input of the compressor is equal to the enthalpy difference.
Thus, the work input of the compressor per unit mass of the methane using departure charts is
Want to see more full solutions like this?
Chapter 12 Solutions
THERMODYNAMICS
- What is the difference between entropies of oxygen at 150 kPa and 39°C and oxygen at 150 kPa and 337°C on a perunit-mass basis?arrow_forwardCombustion gases enter an adiabatic gas turbine at 1540°F and 120 psia and leave at 60 psia with a low velocity. Treating the combustion gases as air and assuming an isentropic efficiency of 82 percent, determine the work output of the turbine.arrow_forwardAir is compressed by an adiabatic compressor from 95 kPa and 27°C to 600 kPa and 277°C. Assume variable specific heats and neglect the changes in kinetic and potential energies. Determine the isentropic efficiency of the compressor. Use the table containing the ideal-gas properties of air. The isentropic efficiency of the compressor is _______%.arrow_forward
- Five kg of air at 427°C and 600 kPa are contained in a piston–cylinder device. The air expands adiabatically until the pressure is 100 kPa and produces 600 kJ of work output. Assume air has constant specific heats evaluated at 300 K. Determine the entropy change of the air in kJ/kg·K? Using concepts of the second law, support your answer.arrow_forwardThree kg of helium gas at 100 kPa and 27°C are adiabatically compressed to 900 kPa. If the isentropic compression efficiency is 80 percent, determine the required work input and the final temperature of helium.arrow_forwardArgon gas enters an adiabatic turbine at 1350°F and 200 psia at a rate of 40 lbm/min and exhausts at 20 psia. If the power output of the turbine is 105 hp, determine the isentropic efficiency.arrow_forward
- A piston–cylinder device contains 1.2 kg of nitrogen gas at 120 kPa and 27°C.a) The gas is now compressed slowly in a polytropic process during which PV1.3 = constant. The process ends when the volume is reduced by one-half. Determine the final temperature, pressure and entropy change of nitrogen during the process.b) If the gas, instead, undergoes an isentropic compression to one-half of its initial volume, what are the final temperature and pressure? (Please write assumptions)arrow_forwardWhat is the total entropy change to expand water at 30 psia and 70 percent quality to 10 psia in a closed system undergoing an isothermal, reversible process while exchanging heat with an energy reservoir at 350°F? Use data from tables.arrow_forwardWith respect to 1 kg of liquid water:(a) Initially at 0°C, it is heated to 100°C by contact with a heat reservoir at 100°C.What is the entropy change of the water? Of the heat reservoir? What is ΔStotal?(b) Initially at 0°C, it is first heated to 50°C by contact with a heat reservoir at 50°Cand then to 100°C by contact with a reservoir at 100°C. What is ΔStotal?(c) Explain how the water might be heated from 0°C to 100°C so that ΔStotal = 0.arrow_forward
- Liquid methane is commonly used in various cryogenic applications. The critical temperature of methane is 191 K (or –82°C), and thus methane must be maintained below 191 K to keep it in liquid phase. The properties of liquid methane at various temperatures and pressures are given in Table 7–1. Determine the entropy change of liquid methane as it undergoes a process from 110 K and 1 MPa to 120 K and 5 MPa using tabulated properties. What is the error involved in the latter case?arrow_forwardRefrigerant-134a at 700 kPa and 40°C is expanded adiabatically in a closed system to 60 kPa. Determine the work produced, in kJ/kg, and final enthalpy for an isentropic expanFIGURE P7–168 sion efficiency of 80 percent.arrow_forwardWater vapor enters a turbine at 6 MPa and 400C, and leaves the turbine at 100 kPa with the same specific entropy as that at the inlet. Calculate the difference between the specific enthalpy of the water at the turbine inlet and exit.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





