
Use γ-ϕ approach to model the vapor-liquid equilibrium of an ethyne [acetylene] (1) + 1, 1 difluoro ethane [R-152a] (2) system at 303.2 K. Treat the
![Chapter 12.7, Problem 30P, Use - approach to model the vapor-liquid equilibrium of an ethyne [acetylene] (1) + 1, 1 difluoro , example 1](https://content.bartleby.com/tbms-images/9781111580704/Chapter-12/images/80704-12.7-30p-question-digital_image001.png)
liquid using the 2-parameter Margules equation and the vapor as an ideal solution (described by the virial equation). Report the following:
- Raoult’s Law predictions
- Modified Raoult’s Law predictions (ideal gas for the vapor phase)
- γ-ϕ modeling results Experimental data as symbols given in Table P12-30
Table P12-30 Vapor-liquid equilibrium of ethyne (1) + R-152a (2) at 303.2K.
![Chapter 12.7, Problem 30P, Use - approach to model the vapor-liquid equilibrium of an ethyne [acetylene] (1) + 1, 1 difluoro , example 2](https://content.bartleby.com/tbms-images/9781111580704/Chapter-12/images/80704-12.7-30p-question-digital_image002.png)
Interpretation:
Using gamma phi modelling approach, predict the
Concept Introduction:
Write the Antoine equation for the component.
Write the expression to calculate the parameter
Here, gas constant is
Write the expression to calculate the parameter
Write the expression for the van Laar parameters.
Here, temperature is T, parameters of the van Laar equation using the VDW definitions is
Write the activity coefficients from the van Laar model for components 1 and 2.
Here, liquid phase mole fraction for component 1 and 2 is
Write the expression to calculate the value of
Here, reduced temperature of component 1 is
Write the expression to calculate the value of
Here, reduced temperature of component 1 is
Write the expression to calculate the fugacity coefficient of component 1
Write the expression to calculate the fugacity coefficient of component 2
Write the expression for Raoult’s law equation.
Write the expression for the vapor definitions of the fugacity coefficients for component 1 and 2.
Here, system pressure is P, vapor phase mole fraction for components 1 and 2 is
second-virial coefficient of pure component 1 and 2 is
Write the 2-parameter Margules equation.
Here, coefficient of 2-parameter Margules equation is
Write the excess molar Gibbs free energy for model.
Write the excess molar Gibbs free energy for experimental.
Write the objective function value.
Here, total number of data point is N.
Explanation of Solution
Referring to Appendix C.1, “Critical point, enthalpy of phase change, and liquid molar volume”, obtains the critical temperature, critical pressure and acentric factor of Acetylene (1) and 1,1 Difluroethane (2) as in Table (1).
| Compound |
Critical temperature |
Reduced temperature |
Critical pressure |
Acentric factor |
Liquid molar volume |
| Acetylene (1) | 308.3 | 0.9834 | 61.14 | 0.189 | |
| Difluroethane (2) | 386.41 | 0.7846 | 45.17 | 0.275 |
Referring to Appendix E, “Antoine Coefficients”, obtain the constants A, B, and C for component (1) as 4.66141, 909.079, and 7.947 respectively.
Calculate the Antoine equation for the component propane (1).
Substitute
Refer the Appendix E, “Antoine Coefficients”, obtain the constants A, B, and C for component as 7.03000, 910.000, and 244.000 respectively.
Calculate the Antoine equation for the component (2).
Substitute
Calculate the parameter
Here, critical temperature and critical pressure for component 1 are
Substitute 308.3 K for
Calculate the parameter
Here, critical temperature and critical pressure for component 2 are
Substitute 386.41 K for
Calculate the parameter
Substitute 308.3 K for
Calculate the parameter
Substitute 386.41 K for
Calculate the van Laar parameters
Substitute
Calculate the van Laar parameter
Substitute
Write the activity coefficients from the van Laar model for components 1 and 2.
Substitute 0.067713 for
Write the activity coefficients from the van Laar model for components 1 and 2.
Substitute 0.067713 for
Calculate the value of
Substitute 0.9834 for
Calculate the value of
Substitute 0.7846 for
Calculate the value of
Substitute
We know that,
Take up the vapor mole fraction of both the components are equal.
Calculate the saturation pressure for component 1
Substitute 0.5 for
Calculate the saturation pressure for component 2
Substitute 0.5 for
Calculate the fugacity coefficient of component 1
Substitute
Calculate the fugacity coefficient of component 2
Substitute
Express the activity coefficients at the two mixture states.
Here, system pressure is P, vapor phase mole fraction for components 1 and 2 is
Use spreadsheet calculation in Equations (18) and (19) to calculate
| P (bar) | ||||
| 7.01 | 0 | 0 | - | 1.15971297 |
| 7.8 | 0.038 | 0.1572 | 4.50201E+23 | 1.13315852 |
| 8.5 | 0.1283 | 0.3849 | 3.56211E+23 | 0.99665029 |
| 11.75 | 0.2509 | 0.5984 | 3.93668E+23 | 1.05683929 |
| 17.75 | 0.4243 | 0.7603 | 4.51445E+23 | 1.26208752 |
| 25 | 0.5685 | 0.837 | 5.29004E+23 | 1.64767998 |
| 32.49 | 0.6956 | 0.8905 | 6.05561E+23 | 2.08477918 |
| 39.2 | 0.8014 | 0.9378 | 6.75625E+23 | 2.2338378 |
| 47.2 | 0.9003 | 0.9627 | 7.53695E+23 | 3.28987661 |
| 54.76 | 1 | 1 | 8.28467E+23 | - |
Since the compositions are exceptionally close to the immaculate segment endpoints, the activity coefficients are great assessments of the "endless weakening activity coefficients for the components 1 and 2" Thus,
Calculate the 2-parameter Margules equation.
Substitute 2.9832 for
Calculate the 2-parameter Margules equation
Substitute 2.710 for
Calculate the excess molar Gibbs free energy for model.
Calculate the excess molar Gibbs free energy for experimental.
Calculate the objective function value.
Apply spread sheet calculation the objective function value by substituting the
The 2-parameter Margules fit for modified Raoult’s law becomes:
Apply spreadsheet calculation to find the objective function value by substituting the values
of
Calculate the pressure and vapor-stage composition in the accompanying way:
Write the equation of bubble point.
Write the vapor phase mole fraction.
Write the Raoult’s law equation.
Thus, solve each value of
Solve Equations (25) to (30) to find two unknowns
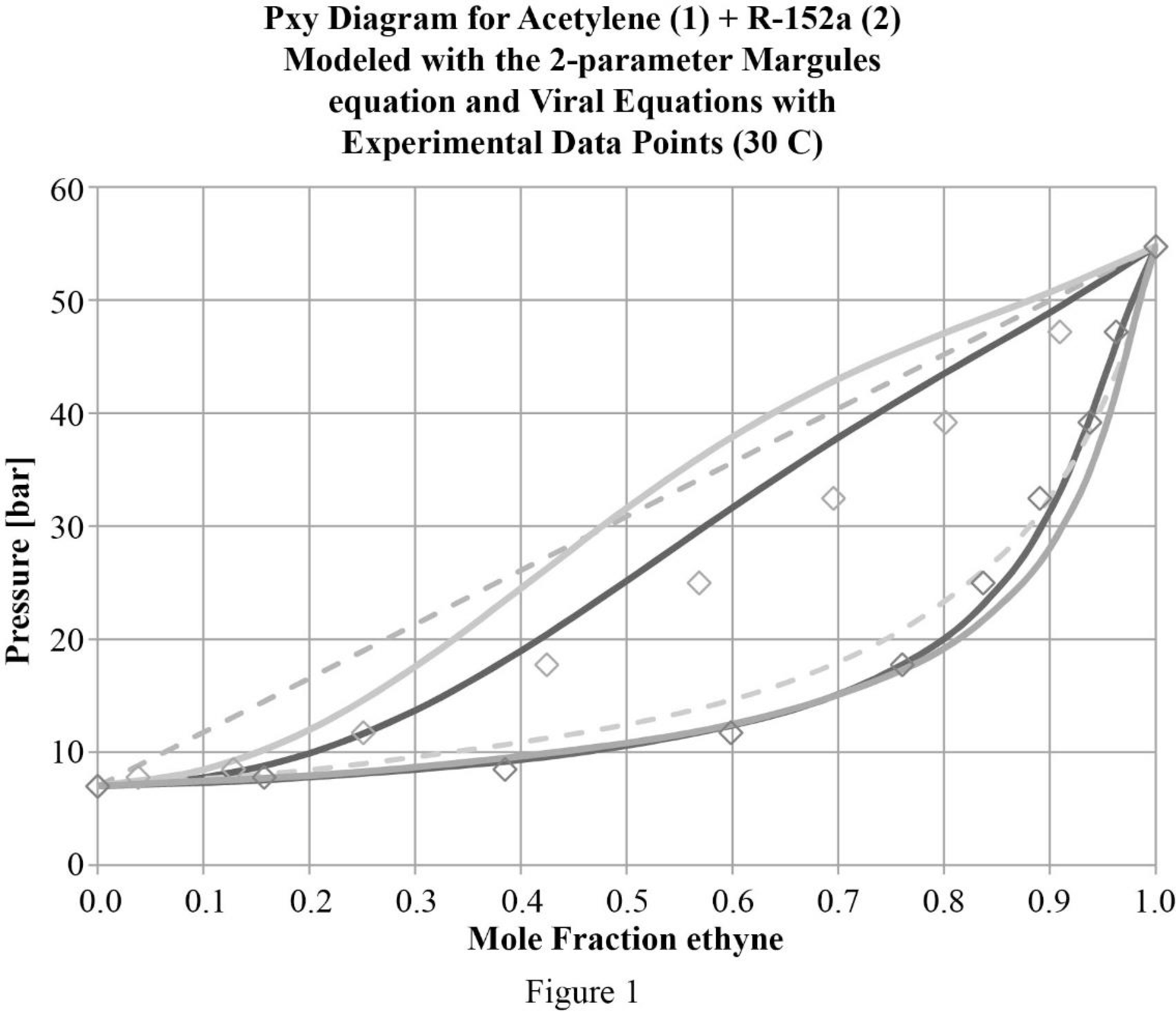
In figure (1) gamma phi approach is indicated by the dark solid line and the modified Raoult’s law is shown by the light solid line and the Raoult’s law by the dashed lines. There is a benefit for treating the system as a real gas instead of as an ideal gas. But at higher pressures, the correlation for bubble point is weaker which is because of the challenge in fitting Gibbs free energy into the model.
Want to see more full solutions like this?
Chapter 12 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





