
Concept explainers
Interpretation:
The Lewis diagram for
Concept introduction:
The Lewis structure shows the connectivity between atoms by identifying the lone pairs of electrons in a compound. Lewis structures are also known as Lewis dot structures. The valence electrons around an atom are shown by dots. Bonds between atoms are shown by lines and the lone pair of electrons is shown by a pair of dots.
Answer to Problem 10E
The Lewis diagram for
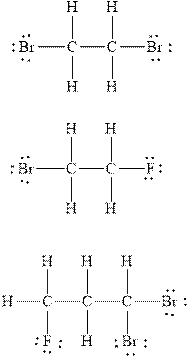
Explanation of Solution
The number of valence electrons in the carbon atom is
In
Therefore, the total number of valence electrons in
These valence electrons are distributed in the Lewis diagram using bonds and lone pair of electrons.
At first, the single bond between the carbon atoms surrounded by other atoms through single bonds is drawn. Each bond contains
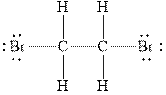
Figure 1
In
Therefore, the total number of valence electrons in
These valence electrons are distributed in the Lewis diagram using bonds and lone pair of electrons.
At first, the single bond between the carbon atoms surrounded by other atoms through single bonds is drawn. Each bond contains
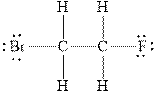
Figure 2
In
Therefore, the total number of valence electrons in
These valence electrons are distributed in the Lewis diagram using bonds and lone pair of electrons.
At first, the single bond between the carbon atoms surrounded by other atoms through single bonds is drawn. Each bond contains
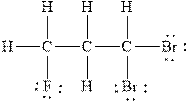
Figure 3
The Lewis diagram for
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- For the Lewis structure presented, answer the following questions. total number of valence electrons______________ e- pair geometry ____________________________ molecular geometry _________________________ HCH angle__________________________________ Polarity __________________________________arrow_forwardFor each of the following molecules draw the Lewis structure on a separate sheet of paper. MAKE SURE TO FOLLOW THE RULES FROM CLASS (ie do not break the octet rule unless necessary to connect all the atoms). These can all be drawn on one piece of paper. Based on your structure provide the following: the total number of valence electrons. a picture of the Lewis structure (3-D model not required). the total number of structural domains around the CENTRAL atom. the number of bonding domains around the CENTRAL atom. the number of lone pairs around the CENTRAL atom. the electronic and molecular shapes. the hybridization of the CENTRAL atom. (sp, sp2, sp3, sp3d, or sp3d2) whether or not the molecule is polar (Enter Y for yes and N for no). Note: The central atom is the first atom listed.arrow_forwardFind the total number of valence electrons, lewis structure, name of electron and molecular geometry, and 3D diagram for SEI2 and SbBr3.arrow_forward
- Using the symbols and +, indicate the direction of polarity in each polar covalent bond. (a) CN (b) NO (c) CClarrow_forwardIndicate whether each of the following hypothetical triatomic molecules is polar or nonpolar. Assume that A, X, and Y have different electronegativities. a. a linear XAX molecule b. a linear XXA molecule c. an angular AXY molecule d. an angular XAY moleculearrow_forwardFill in the blanks in each line of the following table that involves characteristics of various bonds between nonmetals. The first line is already completed as an example.arrow_forward
- Successive substitution of F atoms for H atoms in the molecule CH4 produces the molecules CH3F, CH2F2, CHF3, and CF4. a. Draw Lewis structures for each of the five molecules. b. Using VSEPR theory, predict the geometry of each of the five molecules. c. Specify the polarity (polar or nonpolar) for each of the five molecules.arrow_forwardPlace + above the atom that is relatively positive and above the atom that is relatively negative in each of the following bonds. Try to answer this question without referring to Figure 5-11. a. ClBr b. AlS c. BrS d. ONarrow_forwardDraw Lewis structures showing all valence electrons for these molecules. (a) C2H6 (b) CS2 (c) HCNarrow_forward
- 1. In its Lewis dot symbol, which element would have four (4) dots? Select one: a.Si b.F c.N d.S 2. Which compound is expected to be soluble in a polar solvent? Select one: a.CH4 b.NH3 c.None of these. d.BF3 3. Which compound is nonpolar? Select one: a.CO2 b.None of these. c.HCl d.H2O 4. In HCN, the formal charge on nitrogen is Select one: a.+1 b.0 c.–1 d.None of these. 5. For the carbonate anion, CO3–2, the number of contributing equienergetic resonance structures is Select one: a.3 b.4 c.1 d. 2arrow_forwardPlease help me with number 6. I need the lewis structure, electron geometry, molecular geometry, and lewis structure indicating molecular geometry.arrow_forward[Molecular shape and polarity] Consider two molecules: XCl3, and YO2 X is an atom in group 5A with electronegativity of 2.0. Y is an atom in group 6A with electronegativity of 2.4. Fill in the blanks. Molecule Lewis structure (Draw an lone pair(s) of electrons, when present) Molecular shape Molecular polarity (Yes: polar or No: nonpolar) XCl3 ? ? ? YO2 ? ? ?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





