
Concept explainers
(a)
Interpretation:
Structure of the molecule B should be converted to a condensed structure and IUPAC name of the molecule should be given.
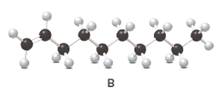
= (
Concept Introduction:
In alkene nomenclature longest C chain will be considered as the parent/ main C chain. At the end of the
Answer to Problem 13.104P
Condensed structure -
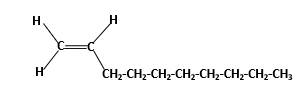
IUPAC name −Decene
Explanation of Solution
In alkenes there is a carbon-carbon double bond and different groups are connected to the two carbons. In most of the alkenes; out of four binding positions of carbon-carbon double bond, two of them are binds with two hydrogen atoms and other two binding positions are binds with two different alkyl groups.In this molecule, carbon atoms are represented in black and hydrogen atoms are represented in white color.Structure of a chemical compound is the arrangement of atoms and bonds in the space.
Condensed structure -
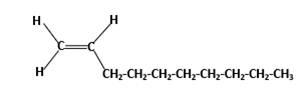
In this molecule, main carbon chain which contains the carbon-carbon double bond is a ten membered chain and there are no substituents in this molecule. According to the numbering of the main chain double bond gets the lowest number and it is number 1.Hence, IUPAC name of the molecule is decene.
(b)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 13.104P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 13.104P
Explanation of Solution
Unsaturated (
Refer to the below reaction;
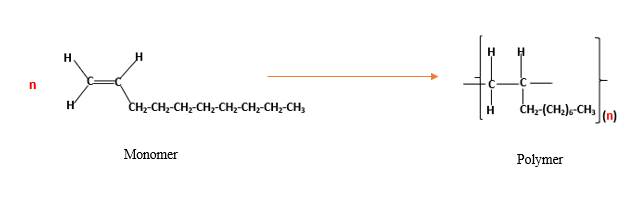
(d)
Interpretation:
The resulting polymer should be identified after
Concept Introduction:
Alkenes are hydrocarbon molecules that contain a carbon-carbon double bond inside a molecule.
Answer to Problem 13.104P
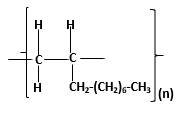
Explanation of Solution
Polymers are high molecular weight macromolecules which formed from covalently bonded monomer molecules. When forming polymers from alkene monomers, the weak bond which is in the carbon-carbon double bond is broken and new strong bond will form to connect the monomer molecules together. Hence; weak bond of the carbon-carbon double bond of
Want to see more full solutions like this?
Chapter 13 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Draw the structures of the following compound and give it's IUPAC name: a. Methyl mercaptan b. sec-BUtyl mercaptan c. 2-Methyl-2-propanethiol d. 1-Pentanethiolarrow_forwardWhat products are formed when benzene is treated with each alkyl chloride and AlCl3?arrow_forwardWhat alkane is formed when each alkene is treated with H2 and a Pd catalyst?arrow_forward
- What alkenes are formed when each alcohol is treated with H 2SO 4? Use the Zaitsev rule to predict the major product.arrow_forwardWhat alcohol is formed when the compound is treated with H2 and a Pd catalyst?arrow_forwardDraw the structure corresponding to each name: a.isobutylbenzene b.o-dichlorobenzene c.cis-1,2-diphenylcyclohexane d.m-bromoaniline e.4-chloro-1,2-diethylbenzene f.3-tert-butyl-2-ethyltoluenearrow_forward
- Please provide your views ? below point are true or false ? a.) Alkenes have low melting points and boiling points.b.) Melting points and boiling points increase as the number of carbonsincreases because of increased surface area.c.) Alkenes are soluble in organic solvents and insoluble in water.arrow_forwardDraw the structure corresponding to each IUPAC name: (a) 5,5-dimethyl-3-heptyne; (b) 1,3-dimethylcyclohexene.arrow_forwardProvide the IUPAC name and structure of the products which result from the following reaction CH2=CH-CH=CH-CH3 with Br2arrow_forward
- 18-Draw the structure corresponding to each IUPAC namearrow_forwardDraw the structure corresponding to each IUPAC name.a. (Z)-4-ethylhept-3-eneb. (E)-3,5,6-trimethyloct-2-enec. (Z)-2-bromo-1-iodohex-1-enearrow_forwardWhat product is formed when p-dimethylbenzene (also called p-xylene) is treated with each reagent?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
