
(a)
Interpretation: An image representing various reactions is given. Various questions based on the given image are to be answered.
Concept introduction: According to the collision theory, for a reaction to take place the molecules must collide in proper orientation and must have minimum energy so that the effective collisions may occur.
To determine: The two images that describe the elementary steps which when combined depicts the destruction of Ozone by Chlorine.
(a)
Answer to Problem 13.12VP
Solution
The two images that describe the elementary steps which when combined depicts the destruction of Ozone by Chlorine are given by figure A and H.
Explanation of Solution
Explanation
The equation for the destruction of Ozone by Chlorine is given as,
The equation (1) is represented by the figure A as shown,
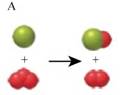
As three red balls correspond to
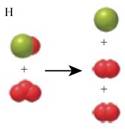
As three red balls correspond to
Conclusion
The two images that describe the elementary steps which when combined depicts the destruction of Ozone by Chlorine are given by figure A and H.
(b)
Interpretation: An image representing various reactions is given. Various questions based on the given image are to be answered.
Concept introduction: According to the collision theory, for a reaction to take place the molecules must collide in proper orientation and must have minimum energy so that the effective collisions may occur.
To determine: The image that represents the overall reaction for the Chlorine catalyzed destruction of Ozone.
(b)
Answer to Problem 13.12VP
Solution
The image that represents the overall reaction for the Chlorine catalyzed destruction of Ozone is given by figure E.
Explanation of Solution
Explanation
The equation for the destruction of Ozone by Chlorine is given as,
The overall reaction is given as,
This situation is represented by figure E as shown below,
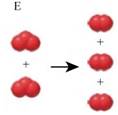
As three red balls combined in reactants correspond to
Conclusion
The image that represents the overall reaction for the Chlorine catalyzed destruction of Ozone is given by figure E.
(c)
Interpretation: An image representing various reactions is given. Various questions based on the given image are to be answered.
Concept introduction: According to the collision theory, for a reaction to take place the molecules must collide in proper orientation and must have minimum energy so that the effective collisions may occur.
To determine: The two images that describe the elementary steps which when combined describes the overall reaction in which
(c)
Answer to Problem 13.12VP
Solution
The two images that describe the elementary steps which when combined describe the overall reaction in which
Explanation of Solution
Explanation
The reaction involving
The equation (3) is represented by figure D as shown below,
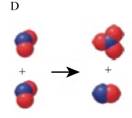
Here, a pair of combined red and blue balls corresponds to
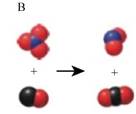
Here, three combined red balls along with one blue ball corresponds to
Conclusion
The two images that describe the elementary steps which when combined describe the overall reaction in which
(d)
Interpretation: An image representing various reactions is given. Various questions based on the given image are to be answered.
Concept introduction: According to the collision theory, for a reaction to take place the molecules must collide in proper orientation and must have minimum energy so that the effective collisions may occur.
To determine: The chemical equation for the reaction described in question (c).
(d)
Answer to Problem 13.12VP
Solution
The chemical equation for the reaction described in question (c) is,
Explanation of Solution
Explanation
The reaction involving
The chemical equation for the overall reaction is given by adding equation (3) and (4) as,
Conclusion
The chemical equation for the reaction described in question (c) is,
(e)
Interpretation: An image representing various reactions is given. Various questions based on the given image are to be answered.
Concept introduction: According to the collision theory, for a reaction to take place the molecules must collide in proper orientation and must have minimum energy so that the effective collisions may occur.
To determine: The image that shows photodecomposition of Chlorofluorocarbons.
(e)
Answer to Problem 13.12VP
Solution
The image that shows photodecomposition of Chlorofluorocarbons is given by figure G.
Explanation of Solution
Explanation
The decomposition of Chlorofluorocarbons is given as,
This equation is represented by figure G as shown below.
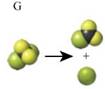
As four combined green and one black ball at left hand side corresponds to
Conclusion
The image that shows photodecomposition of Chlorofluorocarbons is given by figure G.
(f)
Interpretation: An image representing various reactions is given. Various questions based on the given image are to be answered.
Concept introduction: According to the collision theory, for a reaction to take place the molecules must collide in proper orientation and must have minimum energy so that the effective collisions may occur.
To determine: The decreasing order for the collision between Ozone and chlorine atoms.
(f)
Answer to Problem 13.12VP
Solution
The decreasing order for the collision between Ozone and chlorine atoms is
Explanation of Solution
Explanation
The absence of Ozone is represented by the blue cloud. The cloud is bigger in figure I, it means Ozone is most depleted in figure I and it corresponds to the maximum number of collisions between Ozone and Chlorine atoms as shown below,
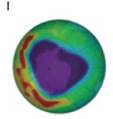
Then figure F shows the less depletion of Ozone in comparison to figure I as shown below,

The least depletion of Ozone is shown in figure C as shown below,

Therefore, the decreasing number of collisions is given by
Conclusion
The decreasing order for the collision between Ozone and chlorine atoms is
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry: The Science in Context (Fifth Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





