
Concept explainers
What are the major IR absorptions in the
a.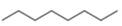 d.
d.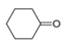
b.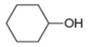
c. 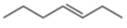 e.
e. 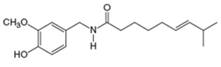
capsaicin
(spicy component of hot peppers)
(a)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorption peaks are observed for
Explanation of Solution
The given compound is octane. It contains
The major IR absorption peaks are observed for
(b)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is cyclohexanol. It contains
The major IR absorptions are observed for
(c)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is hept-3-ene. It contains
The major IR absorptions are observed for
(d)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is cyclohexanone. It contains
The major IR absorptions are observed for
(e)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is capsaicin as shown below.
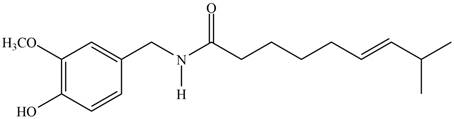
Figure 1
It contains
The major IR absorptions are observed for
Want to see more full solutions like this?
Chapter 13 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
Additional Science Textbook Solutions
Chemistry
Essential Organic Chemistry (3rd Edition)
Organic Chemistry
Inorganic Chemistry
Introduction to Chemistry
General, Organic, & Biological Chemistry
- Explain why the pKa of compound A is lower than the pKa's of bothcompounds B and C.arrow_forwarddraw the structure of anthracene on the IR spectrum and identify the absorption signals for aromatic C=C and aromatic C-H directly on IR spectrumarrow_forwardThe IR and NMR are for trans-p-anisalacetophenone, p-anisaldehyde, and acetophenone. What are the major peaks for each of the IR spectrum and assign the hydrogen peaks on each HNMR. What are the differences in the IR spectrum for the reactants and products?arrow_forward
- Identify each compound from its spectral data.arrow_forward(a) Which column would you use for the separation of compounds A and B. Brieflyexplain the reason for your choice.arrow_forwardHow many peaks are observed in the 1H NMR signal for each proton shown in red in palau'amine, the complex chapteropening molecule?arrow_forward
- a. Which compound has the stretching vibration for its carbonyl group at the highest frequency: acetyl chloride, methyl acetate, or acetamide?b. Which one has the stretching vibration for its carbonyl group at the lowest frequency?arrow_forwardHow many 1H NMR signals does attached compound show ?arrow_forwardAnnotate the IR spectra by labelling each of the major peaks as a functional group. Determine and draw the uknown structure based on the IR spectra.arrow_forward
