
Write structural formulas for the following compounds.
- (a) C2H4Br2: δ 2.5 (d, 3H) and 5.9 (q, 1H)
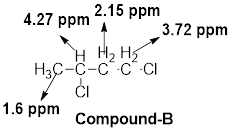
- (b) C4HgCl2: δ 1.60 (d, 3H), 2.15 (m, 2H), 3.72 (t, 2H), and 4.27 (m, 1H)
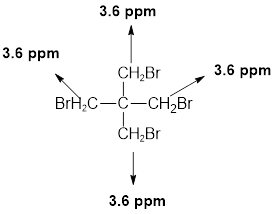
- (c) C5H8Br4: δ 3.6 (s, 8H)
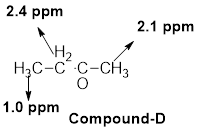
- (d) C4H8O: δ 1.0 (t, 3H), 2.1 (s, 3H), and 2.4 (quartet, 2H)
- (e) C4H8O2: δ 1.2 (t, 3H), 2.1 (s, 3H), and 4.1 (quartet, 2H); contains an ester
- (f) C4H8O2: δ 1.2 (t, 3H), 2.3 (quartet, 2H), and 3.6 (s, 3H); contains an ester
- (g) C4H9Br: δ 1.1 (d, 6H), 1.9 (m, 1H), and 3.4 (d, 2H)
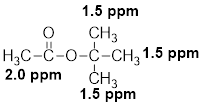
- (h) C6H12O2: δ 1.5 (s, 9H) and 2.0 (s, 3H)
- (i) C7H14O: δ 0.9 (t, 6H), 1.6 (sextet, 4H), and 2.4 (t, 4H)
- (j) C5H10O2: δ 1.2 (d, 6H), 2.0 (s, 3H), and 5.0 (septet, 1H)
- (k) C5H11Br: δ 1.1 (s, 9H) and 3.2 (s, 2H)
- (l) C7H15Cl δ 1.1 (s, 9H) and 1.6 (s, 6H)
(a)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (a) is
There are 2 peaks observed in the given
A doublet is observed for 3 hydrogens at around
Another quartet peak for 1 hydrogen at around
Based on the above
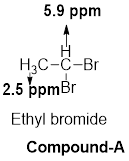
(b)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (b) is
A doublet is observed for 3 hydrogens at around
A multiplet is observed for 2 hydrogens at around
One triplet peak is observed for 2 hydrogens at around
Another multiplet is observed for 1 hydrogen at around
Thus, the structure of the compound (b) is 1,3-dichlorobutane:
(c)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (c) is
This molecule only one singlet is observed for 8 hydrogens at around
Therefore, the structure of the compound (c) is given below and this molecule symmetrical.
(d)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (d) is
A triplet is observed for 3 hydrogens at around
One singlet is observed for 3 hydrogens at around
Another quartet is observed for 2 hydrogens at around
Thus, the structure of the compound (d) is,
(e)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (e) is
A triplet is observed for 3 hydrogens at around
One singlet is observed for 3 hydrogens at around
Another quartet is observed for 2 hydrogens at around
Thus, the structure of the compound (e) is,
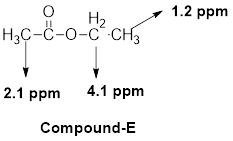
(f)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (f) is
A triplet is observed for 3 hydrogens at around
One singlet is observed for 3 hydrogens at around
Another quartet is observed for 2 hydrogens at around
Thus, the structure of the compound (f) is,
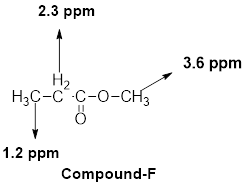
(g)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (g) is
A doublet is observed for 6 hydrogens at around
One multiplet is observed for 1 hydrogen at around
Another multiplet is observed for 2 hydrogens at around
Thus, the structure of the compound (g) is,
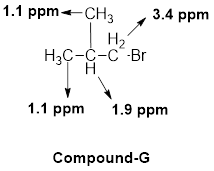
(h)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (h) is
A singlet is observed for 9 hydrogens at around
Another one singlet is observed for 3 hydrogens at around
Thus, the structure of the compound (h) is,
(i)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
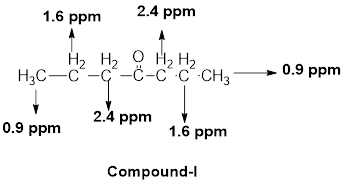
The given molecular formula (i) is
A singlet is observed for 6 hydrogens at around
One sextet is observed for 4 hydrogens at around
Another triplet peak for 4 hydrogens at around
Thus, the structure of the compound (i) is,
(j)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
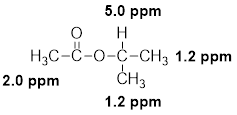
The given molecular formula (J) is
A doublet is observed for 6 hydrogens at around
One septet is observed for 1 hydrogen at around
Another singlet peak for 3 hydrogens at around
Thus, the structure of the compound (J) is,
(k)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (K) is
A singlet is observed for 9 hydrogens at around
One singlet is observed for 2 hydrogens at around
Thus, the structure of the compound (K) is,
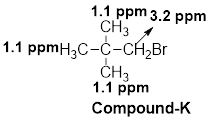
(l)
Interpretation:
The structural formula of the given compound has to be proposed with the help of given molecular formula and its
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
The given molecular formula (L) is
A singlet is observed for 9 hydrogens at around
One singlet is observed for 6 hydrogens at around
Thus, the structure of the compound (L) is,
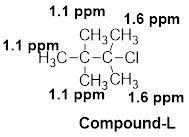
Want to see more full solutions like this?
Chapter 13 Solutions
ORG.CHEM:TXT+OWLV2+MINDTAP 6MTHS >BI<
Additional Science Textbook Solutions
General, Organic, & Biological Chemistry
Elementary Principles of Chemical Processes, Binder Ready Version
General Chemistry: Atoms First
Chemistry: Atoms First
Basic Chemistry (5th Edition)
- The German chemist Wilhelm Kӧrner (1839-1925) observed in 1974 that each of the three isomers of dibromobenzene, A, B, and C, gave a different number of tribromobenzenes upon further bromination, allowing him to assign their respective structures. Try to do the same and assign structures to A, B, and C based on the following results: A gives two tribromobenzenes in comparable amounts B gives three tribromobenzenes, one of them in minor quantities C gives only one tribromobenzenearrow_forwardCompound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forwardFollowing are the 13C and 1H spectra for one of four isomeric bromoalkanes with formula C4H9Br. Assign a structure of the isomer.arrow_forward
- the compound B C23H46,a sex attractant of the common housefly,gave two products Ch3(cH2)12COOH and CH3(CH2)7COOH. Based on this information, suggest the likely structures of Barrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forwardA compound C4H11N is known from its reactivity andspectroscopic properties to have no hydrogen atomsattached directly to the nitrogen atom. Write all structuralformulas consistent with this informationarrow_forward
- Compound X has molecular formula C5H10. In the presence of a metal catalyst, compound X reacts with one equivalent of molecular hydrogen to yield 2-methylbutane. (1) Suggest three possible structures for compound X. (2) Hydroboration-oxidation of compound X yields a product with no chirality centers. Identify the structure of compound X.arrow_forwardThe NMR spectrum of bromocyclohexane indicates a low field signal (1H) at δ 4.16. To room temperature, this signal is a singlet, but at -75 ° C it separates into two peaks of unequal area (but totaling one proton): δ 3.97 and δ 4.64, in ratio 4.6: 1.0. How do you explain the doubling in two peaks? According to the generalization of the previous problem, what conformation of the molecule predominates (at -75 ° C)? What percentage of the molecules does it correspond to? Solve all parts otherwise down vote and hand written solutionarrow_forwardCalculate the index of hydrogen deficiency of this compound Q.) Ascorbic acid (vitamin C), C6H8O6arrow_forward
- The following 1H NMR spectra are for four compounds, each with molecular formula of C6H12O2. Identify the compoundsarrow_forwardAnts emit tiny amounts of chemicals called alarm pheromones to warn other ants (of the same species) of the presence of an enemy. Several of the components of the pheromone in one species have been identified, and two of their structures follow. Which compound has the infrared spectrum shown? -0 -O Citral Citronellal 12025 1720 cm"1 2. The main constituent of cinnamon oil has the formula C9H8O. From the following infrared spectrum, deduce the structure of this component. 1677 cm 1200 1000 3. The infrared spectra of cis- and trans-3-hexen-1-ol follow. Assign a structure to each spectrum.arrow_forwardCompound A, C5H10O, is one of the basic building blocks of nature. All steroids ans many other naturally occurring compounds are built from compound A. Spectroscopic analysis of A yields the following information. a) how many double bonds and/or rings does A have? b) From the IR spectrum, what is the identity of the oxygen-conataining functional group? c) what kinds of protons are responsible for the NMR absorptions listed d) propose a structure for Aarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

