
(a)
Interpretation:
The parameter values for the Margules equation which provide the best fit of
Concept Introduction:
Equation
The formula to calculate the value of
Margules equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
Here,
The equations used to calculate
(a)
Answer to Problem 13.34P
The parameter values for the Margules equation which provide the best fit of
The
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol (1)/water (2) at
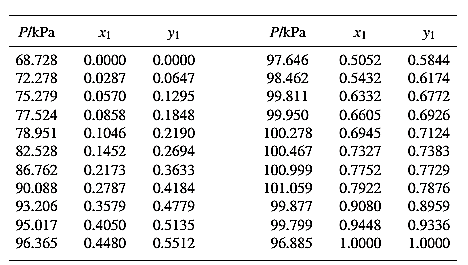
From the given data, first calculate the value of
Now, use equation (1) for the Modifies Raoult’s law and calculate the value of
Use the equation (2) to calculate the value of
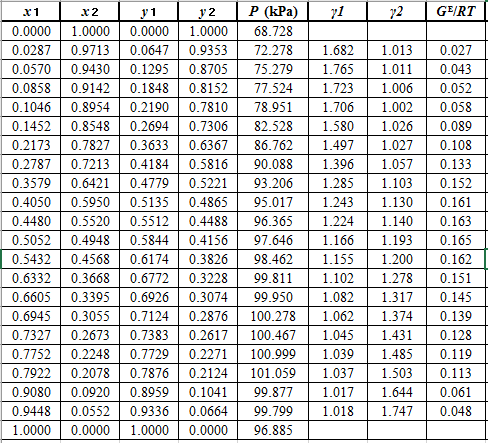
Rewrite equation (3) so that the equation becomes linear in
Now, calculate the values of
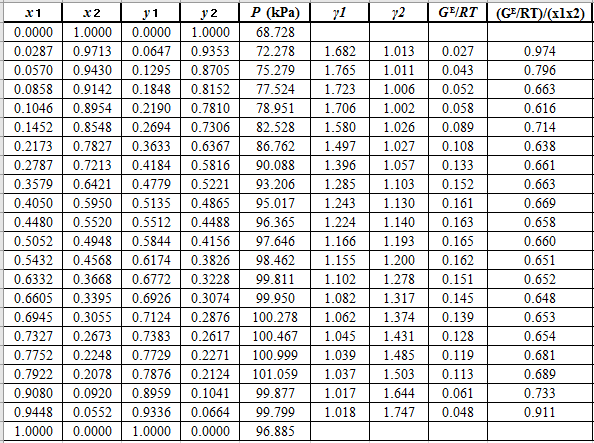
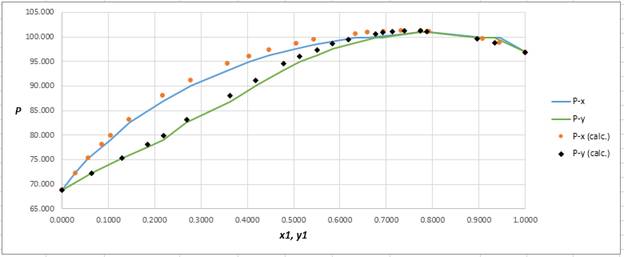
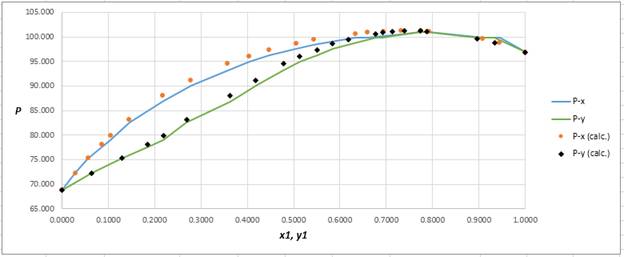
Plot the graph of
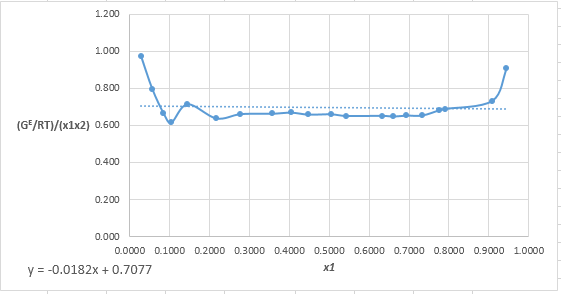
The equation that fits the plot is:
Compare it with equation (5) so that the values of
According to the above correlation for
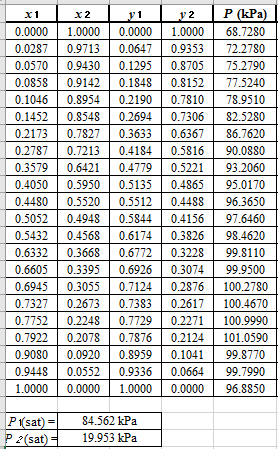
Now, using the above relations, calculate the values of
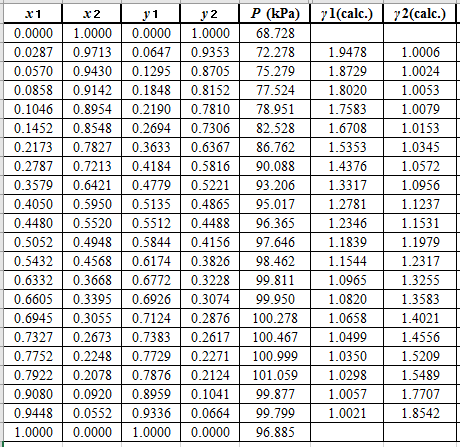
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of
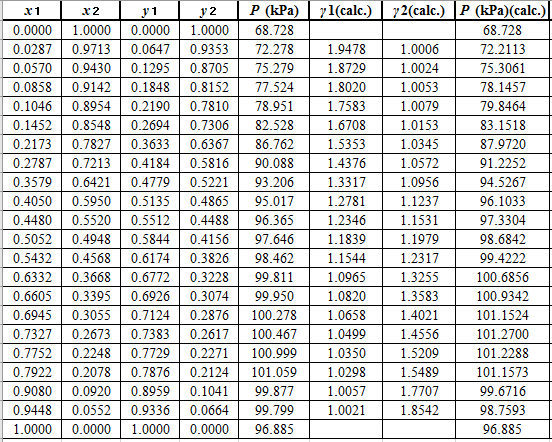
Now, use the below mentioned formula to calculate the value of
Using the tools of the excel, plot the graph of
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
(b)
Interpretation:
The parameter values for the van Laar equation which provide the best fit of
Concept Introduction:
Equation
The formula to calculate the value of
Van Laar equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
Here,
The equations used to calculate
(b)
Answer to Problem 13.34P
The parameter values for the van Laar equation which provide the best fit of
The
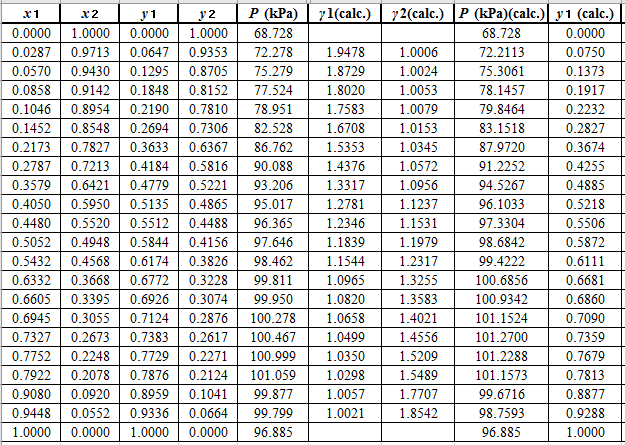
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol (1)/water (2) at
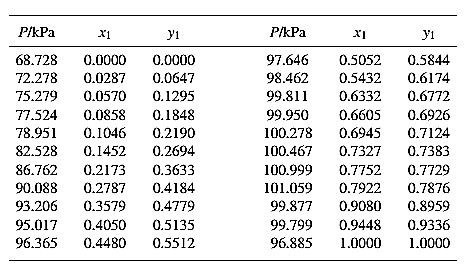
From the given data, first calculate the value of
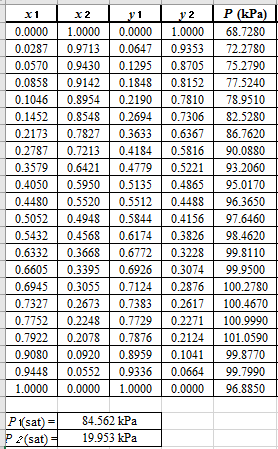
Now, use equation (1) for the Modifies Raoult’s law and calculate the value of
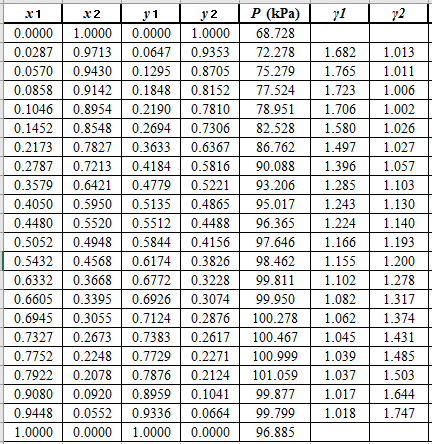
Use the equation (2) to calculate the value of
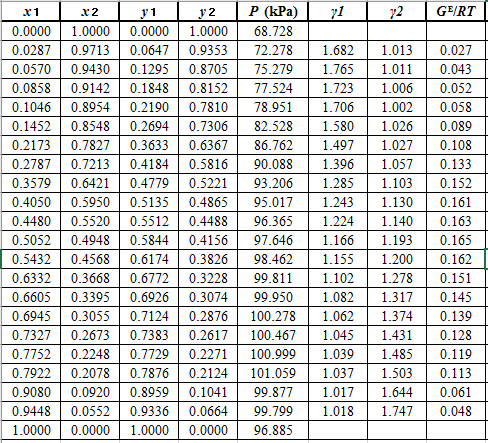
Rewrite equation (6) so that the equation becomes linear in
Now, calculate the values of
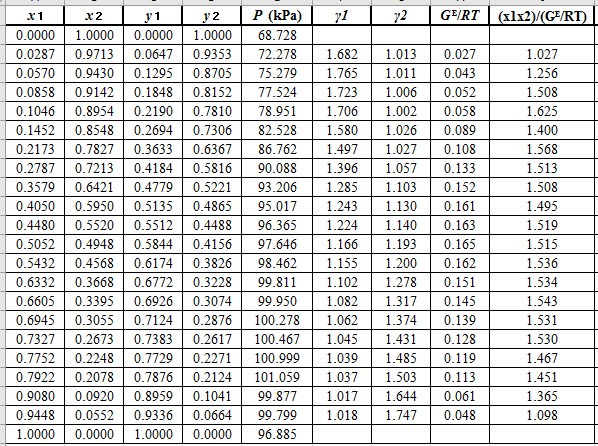
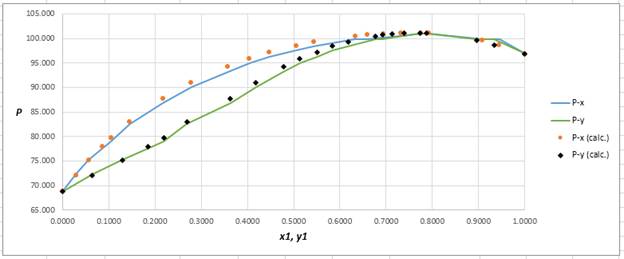
Plot the graph of
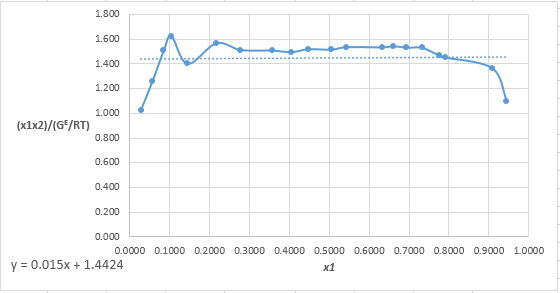
The equation that fits the plot is:
Compare it with equation (8) so that the values of
According to the above correlation for
Now, using the above relations, calculate the values of
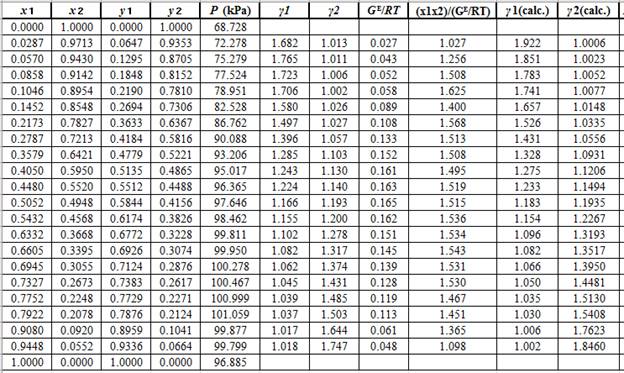
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of
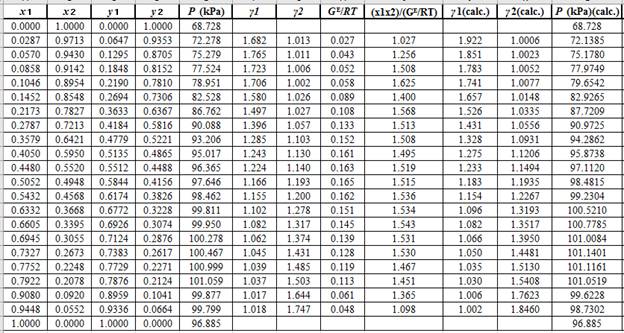
Now, use the below mentioned formula to calculate the value of
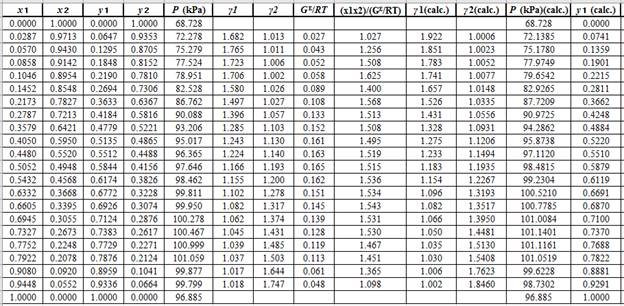
Using the tools of the excel, plot the graph of
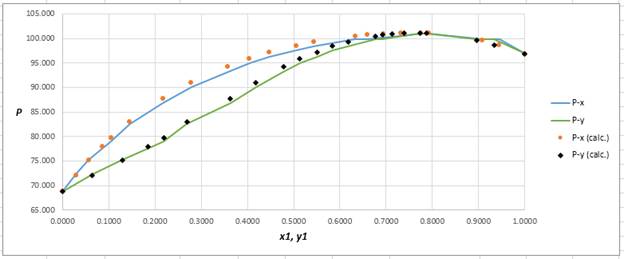
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
(c)
Interpretation:
The parameter values for the Wilson equation which provide the best fit of
Concept Introduction:
Equation
The formula to calculate the value of
Wilson equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
Here,
The equations used to calculate
(c)
Answer to Problem 13.34P
The parameter values for the Wilson equation which provide the best fit of
The
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at
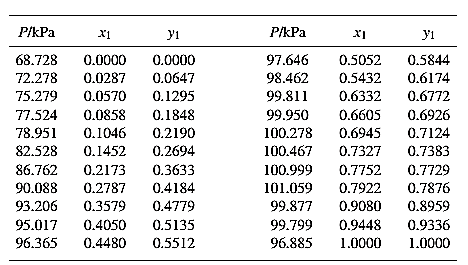
From the given data, first calculate the value of
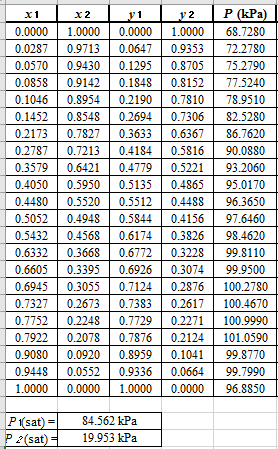
Now, use equation (1) for the Modifies Raoult’s law and calculate the value of
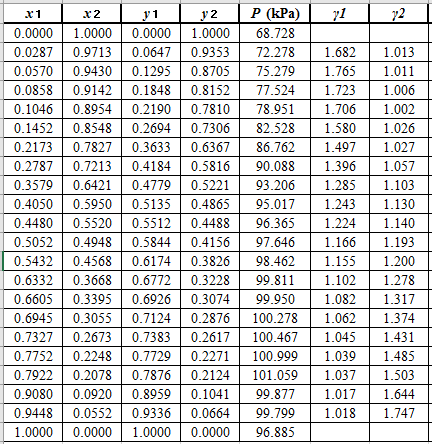
Use the equation (2) to calculate the value of
Now, use the method of the non-linear least square and fit the
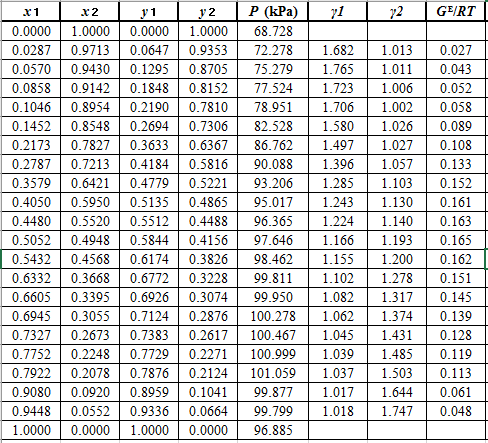
Use the values of
Now, using the above relations, calculate the values of
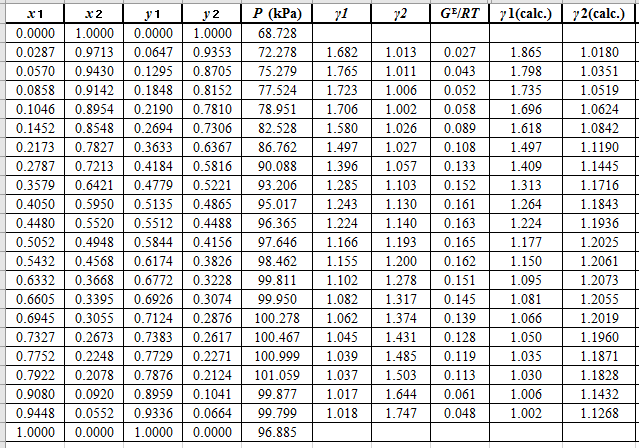
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of
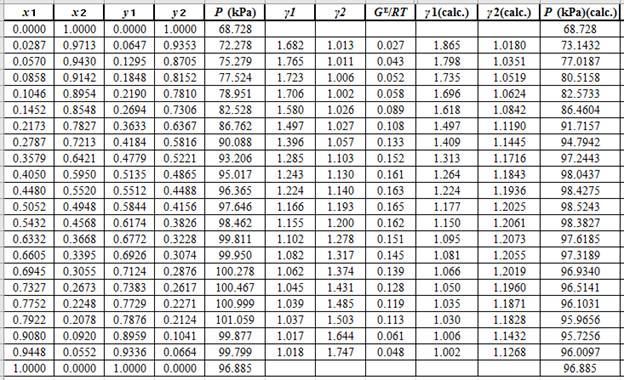
Now, use the below mentioned formula to calculate the value of
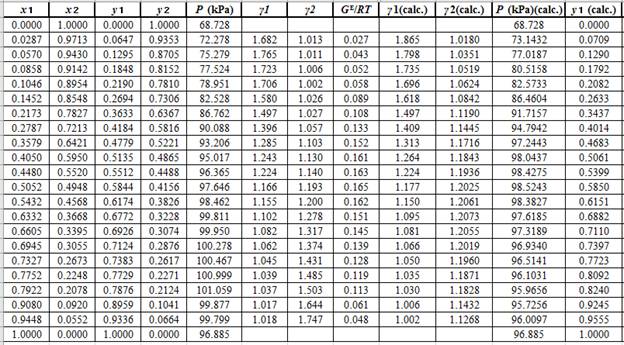
Using the tools of the excel, plot the graph of
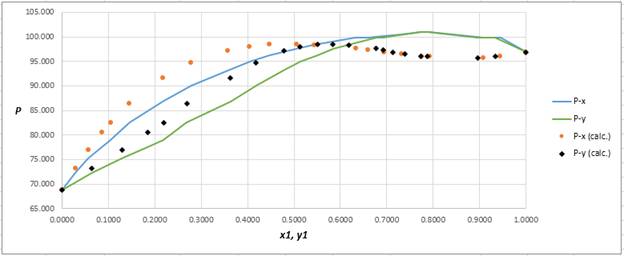
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
Since, the RMS value is considerable, the experimental and calculated value of pressure deviate from each other by a considerable measure.
(d)
Interpretation:
The parameter values for the Margules equation which provide the best fit of
Concept Introduction:
Equation
Margules equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
Here,
The equations used to calculate
(d)
Answer to Problem 13.34P
The parameter values for the Margules equation which provide the best fit of
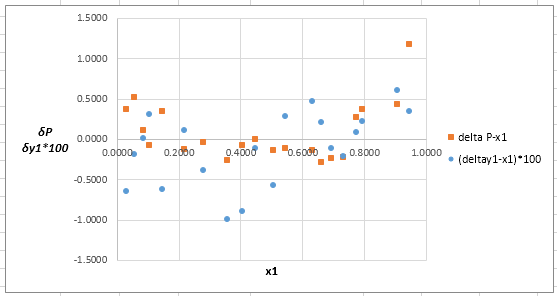
The plot residuals
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at
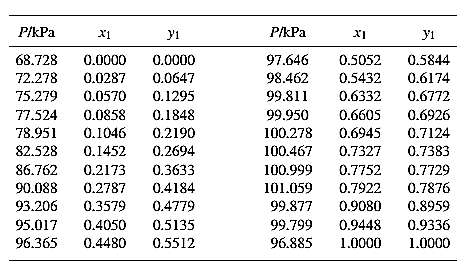
Barker’s Method is the method of determining the parameters by non-linear least squares.
Guess an initial value of
Now, calculate the value of
Then find the sum of the squared errors (SSE) using the below mentioned formula and minimize this value to get the value of the parameters
Using the equation set (4) and the parameter values, deduce the relation for
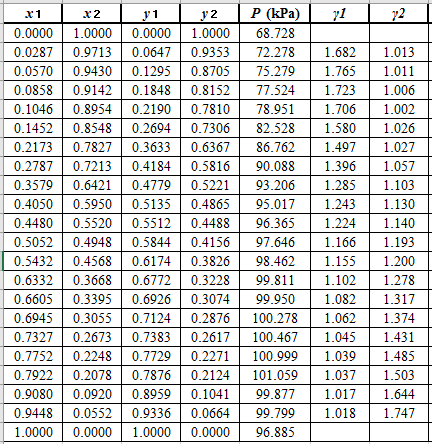
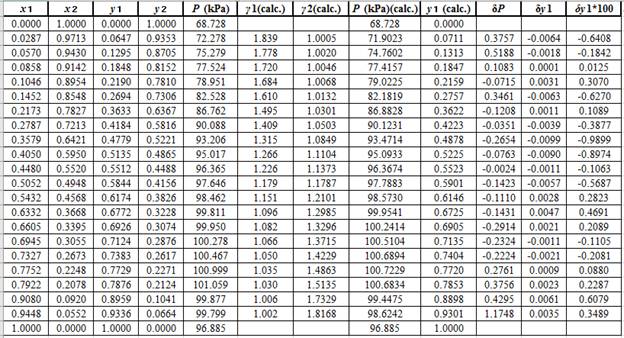
Now, using the above relations, calculate the values of
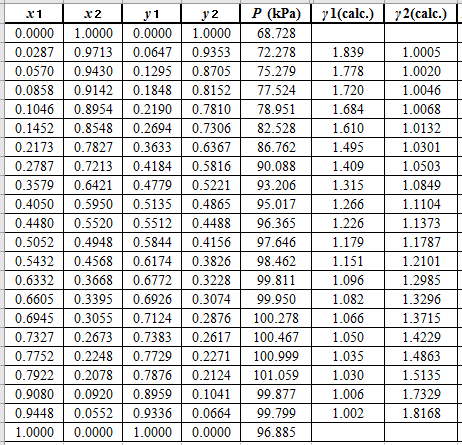
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of
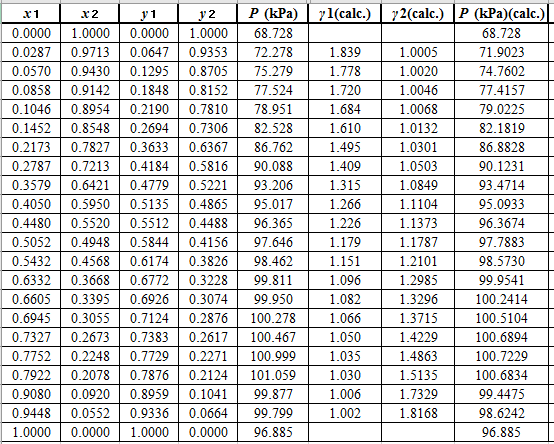
Now, use the below mentioned formula to calculate the value of
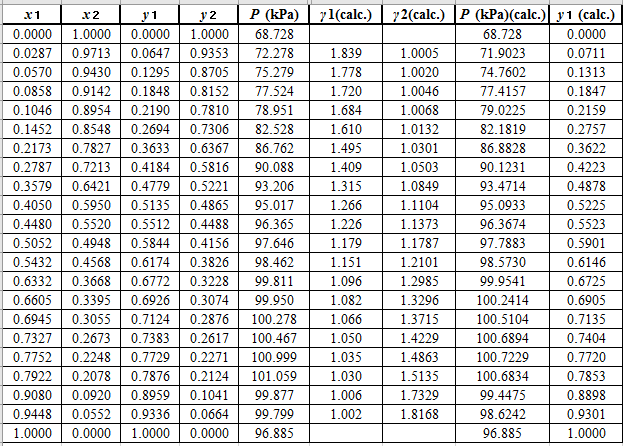
Using the tools of the excel, plot the graph of
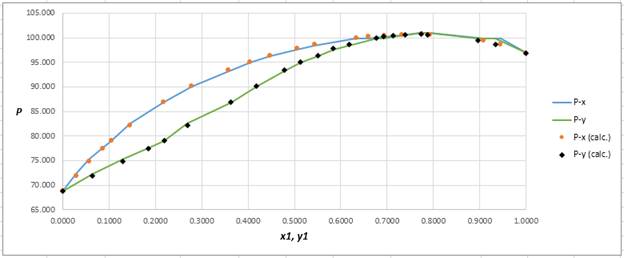
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
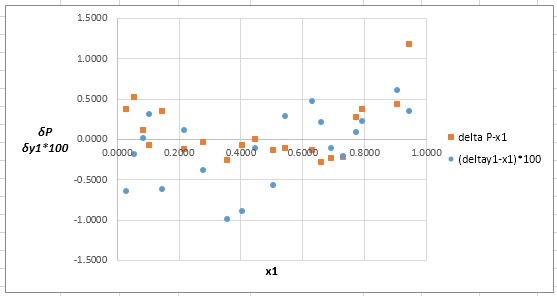
Now, calculate the residuals
Plot these residuals against
(e)
Interpretation:
The parameter values for the van Laar equation which provide the best fit of
Concept Introduction:
Equation
Van Laar equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
Here,
The equations used to calculate
(e)
Answer to Problem 13.34P
The parameter values for the van Laar equation which provide the best fit of
The plot residuals
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol (1)/ water (2) at
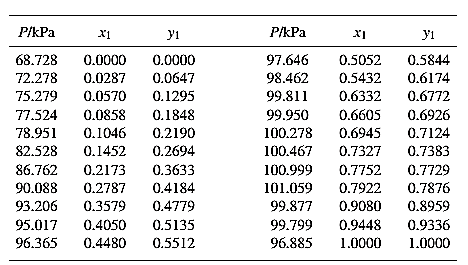
Barker’s Method is the method of determining the parameters by non-linear least squares.
Guess an initial value of
Now, calculate the value of
Then find the sum of the squared errors (SSE) using the below mentioned formula and minimize this value to get the value of the parameters
Using the equation set (7) and the parameter values, deduce the relation for
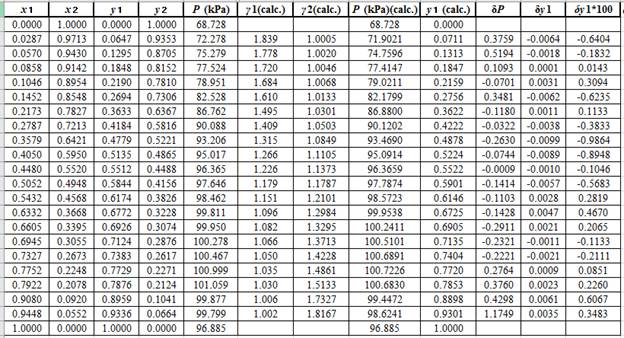
Now, using the above relations, calculate the values of
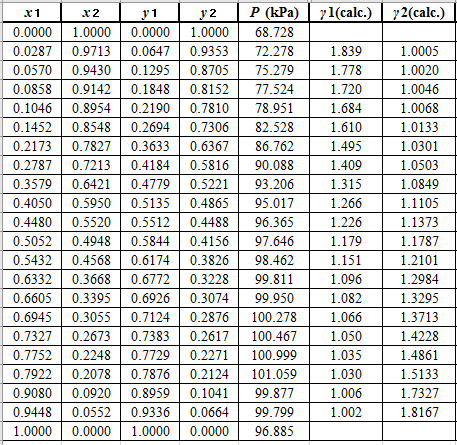
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of
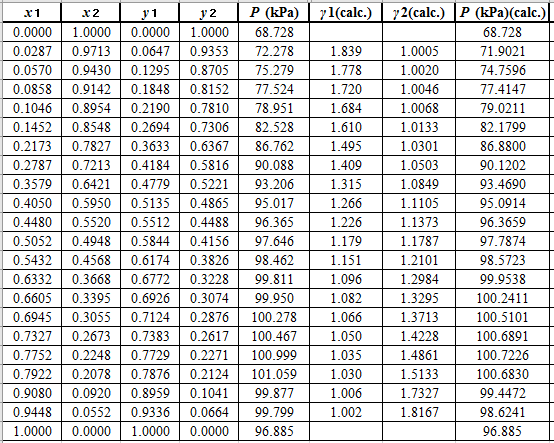
Now, use the below mentioned formula to calculate the value of
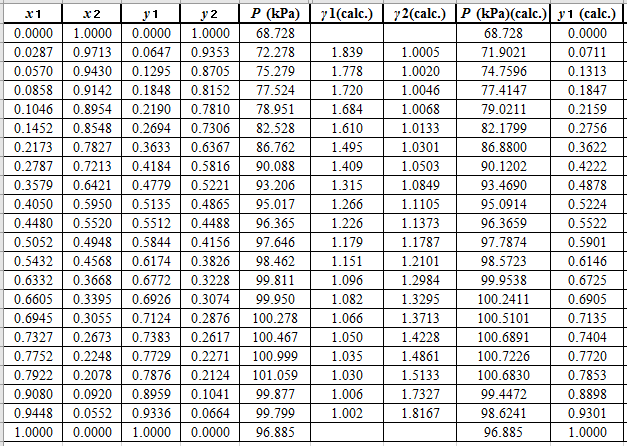
Using the tools of the excel, plot the graph of
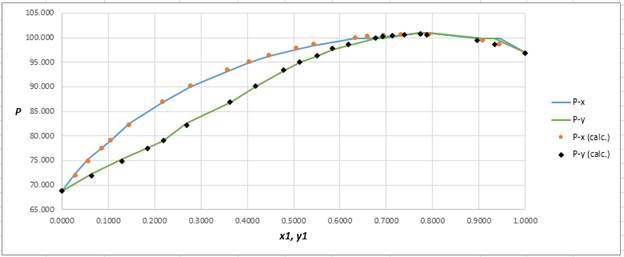
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
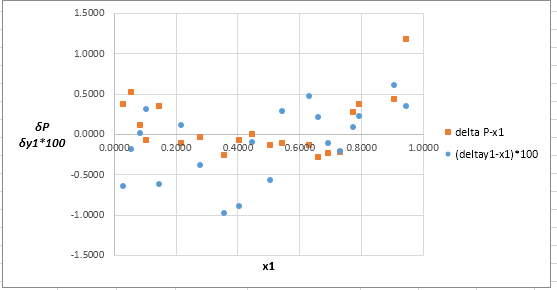
Now, calculate the residuals
Plot these residuals against
(f)
Interpretation:
The parameter values for the Wilson equation which provide the best fit of
Concept Introduction:
Equation
Wilson equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
Here,
The equations used to calculate
(f)
Answer to Problem 13.34P
The parameter values for the Wilson equation which provide the best fit of
The plot residuals
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol (1)/water (2) at
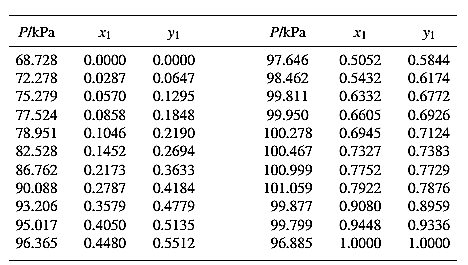
Barker’s Method is the method of determining the parameters by non-linear least squares.
Guess an initial value of
Now, calculate the value of
Then find the sum of the squared errors (SSE) using the below mentioned formula and minimize this value to get the value of the parameters
Using the equation set (10) and the parameter values, deduce the relation for
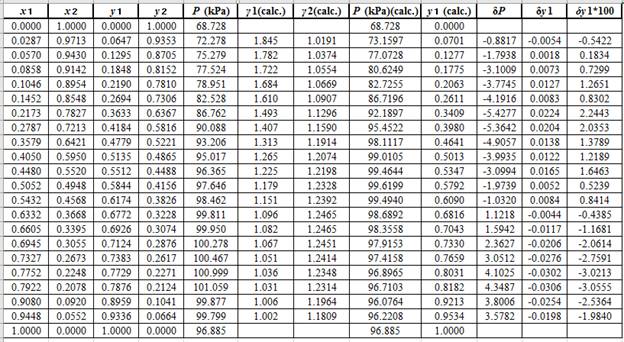
Now, using the above relations, calculate the values of
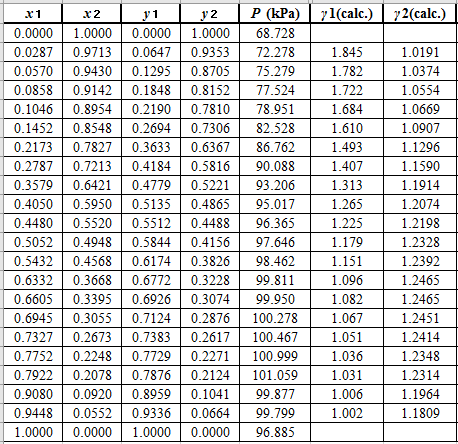
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of
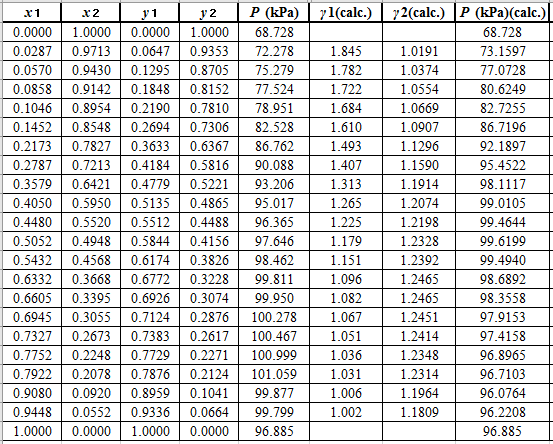
Now, use the below mentioned formula to calculate the value of
Using the tools of the excel, plot the graph of
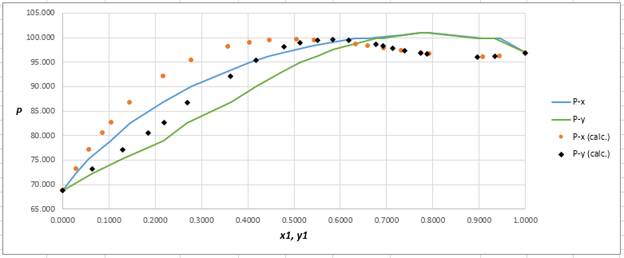
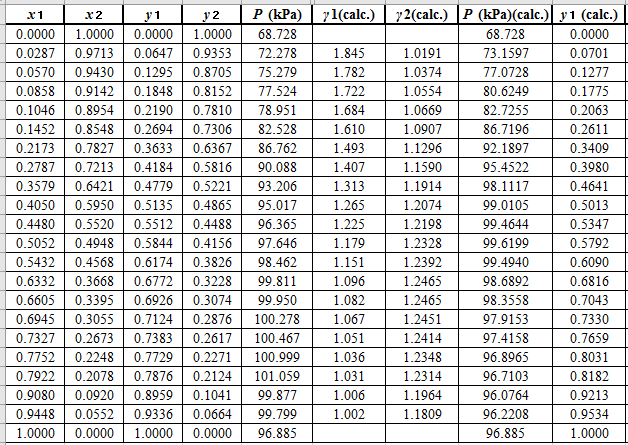
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
Since, the RMS value is considerable, the experimental and calculated value of pressure deviate from each other by a considerable measure.
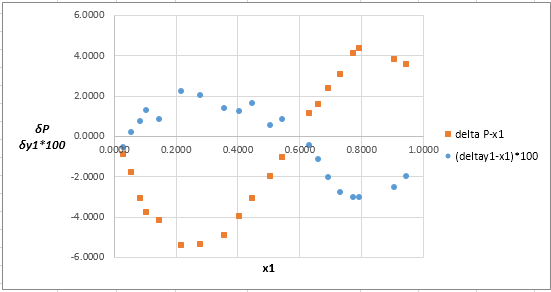
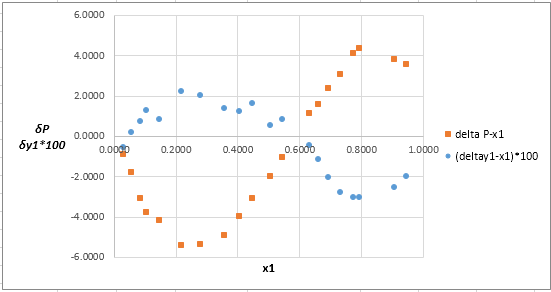
Now, calculate the residuals
Plot these residuals against
Want to see more full solutions like this?
Chapter 13 Solutions
GEN, ORG & BIOL CHEM: CUSTOM SSC
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





