
Concept explainers
(a)
Interpretation:
The structural formulas for lycopene and
Concept Introduction:
Lycopene and
Allura Red and Sunset Yellow are colorants.
Allura Red:
The structure of Allura Red is as follows.
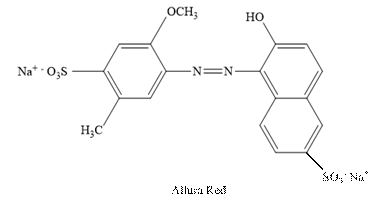
Sunset Yellow:
The structure of Sunset is as follows.
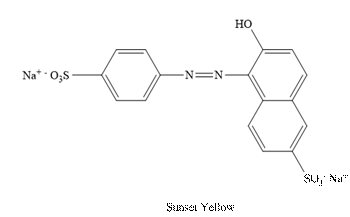
(b)
Interpretation:
The structural feature which is common among Lycopene,
Concept Introduction:
Lycopene and
Allura Red and Sunset Yellow are colorants.
Allura Red:
The structure of Allura Red is as follows.
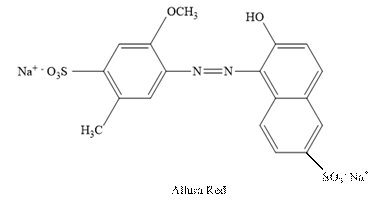
Sunset Yellow:
The structure of Sunset is as follows.
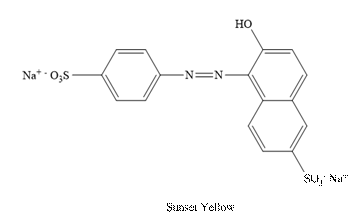
(c)
Interpretation:
Explain why Lycopene,
Concept Introduction:
The compounds which have polar groups are soluble in water.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Introduction to General, Organic and Biochemistry
- 13-52 2,6-Di-/ezY-butyl-4-methylphenol (BHT, Section 13-4C) is an antioxidant added to processed foods to “retard spoilage.” How does BHT accomplish this goal?arrow_forward13-57 Benzene, as we have seen in this chapter, is the simplest aromatic compound. Pyidine is an analog of benzene in which a CH group is replaced by a nitrogenarrow_forward13-56 Four alternatives to the structure of benzene proposed by Kekule are shown in the text. In this problem, we ask you to propose additional alternatives for compounds with the molecular formula C6Hg. As you think about possible alternatives keep in mind the building blocks available to you. Also keep in mind that carbon must be tetravalent, that is, each carbon must have no more and no fewer than four bonds. Three- four-, five-, and six-membered rings Carbon—carbon single, double, and triple bonds The possibility for cis /trans isomerism for carbon-carbon double bondsarrow_forward
- 13-11 Explain why the compound 1,4-dichlorobenzene does not show eis-trans isomerism.arrow_forward13-38 (Chemical Connections 13A) What is meant by the term biodegradable?arrow_forward13-20 Three products with the molecular formula CgH^BrCl form when bromobenzene is treated with chlorine, Cl2, in the presence of FeCl3 as a catalyst. Name and draw a structural formula for each product.arrow_forward
- 13-43 (Chemical Connections 13E) Which features of Allura Red and Sunset Yellow make them water-soluble?arrow_forward13-19 Suppose you have unlabeled bottles of benzene and cyclohexene. What chemical reaction could you use to tell which bottle contains which chemical? Explain what you would do, what you would expect to see, and how you would explain your observations. Write an equation for a positive test.arrow_forward12-64 (Chemical Connections 120 What is the molecular formula of 11-tetradecenyl acetate? What is its molecular weight?arrow_forward
- 12-57 Hydrocarbon A, Cf,Hs, reacts with 2 moles of Br2to give l,2,3,4-tetrabromo-2-methylbutane. What is the structure of hydrocarbon A?arrow_forward13-31 What structural features are common to vitamin E, BHT, and BHA (the three antioxidants presented in Section 13-40?arrow_forward14-66 1,4-Butanediol, hexane, and 1-pentanol have similar molecular weights. Their boiling points, arranged from lowest to highest, are 69°C, 138°C, and 230°C. Which compound has which boiling point?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
