
Concept explainers
Complete the following reactions:
a.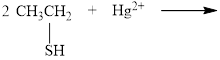
b.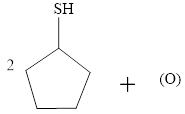
c.
(a)
Interpretation:
The complete reaction between
Concept introduction:
A chemical reaction is a process in which rearrangement of atom or ions takes place between two reacting species. A balanced chemical equation represents an equation in which all the reactants and products are written with their stoichiometric coefficients. The number of atoms of an element on both sides of the equation is equal.
Answer to Problem 13.51E
The completed reaction between
Explanation of Solution
The incomplete reaction is shown below.
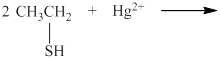
Figure 1
The thiols react with heavy metal ions such as
Therefore, the completed reaction between
The completed reaction between
(b)
Interpretation:
The complete reaction between cyclopentanethiol and nascent oxygen atom is to be stated.
Concept introduction:
A chemical reaction is a process in which rearrangement of atom or ions takes place between two reacting species. A balanced chemical equation represents an equation in which all the reactants and products are written with their stoichiometric coefficients. The number of atoms of an element on both sides of the equation is equal.
Answer to Problem 13.51E
The completed reaction between cyclopentanethiol and the nascent oxygen atom is shown below.
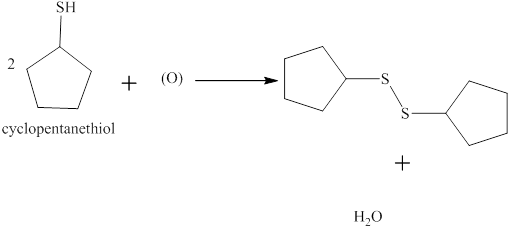
Explanation of Solution
The incomplete reaction is shown below.
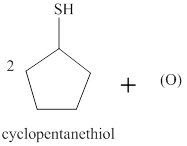
Figure 2
Two molecules of thiol undergo an oxidation reaction with a nascent oxygen atom. The thiol molecules react with a nascent oxygen atom to form a disulfide compound. Therefore, the completed reaction between cyclopentanethiol and the nascent oxygen atom is shown below.

Figure 3
The completed reaction between cyclopentanethiol and the nascent oxygen atom is shown in Figure 3.
(c)
Interpretation:
The complete reaction between
Concept introduction:
A chemical reaction is a process in which rearrangement of atom or ions takes place between two reacting species. A balanced chemical equation represents an equation in which all the reactants and products are written with their stoichiometric coefficients. The number of atoms of an element on both sides of the equation is equal.
Answer to Problem 13.51E
The complete reaction between
Explanation of Solution
The incomplete reaction is shown below.
A disulfide compound undergoes reduction reaction with a nascent hydrogen atom to form two thiol molecules.
Therefore, the complete reaction between
The complete reaction between
Want to see more full solutions like this?
Chapter 13 Solutions
LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card for Seager/Slabaugh/Hansen’s Chemistry for Today: General, Organic, and Biochemistry, 9th
- Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules.arrow_forwardAlcohols A, B and C all have the composition C4H 100. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde; molecules of alcohol B contain a linear carbon chain and can be oxidized to a ketone; and molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone. Write the Lewis structures of these molecules.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





