
Interpretation:
The reaction when hydrogen chloride reacts with 2-methyl-1,3-butadiene to form 1-chloro-3-methyl-2-butene as a major product via 1,4-addition mechanism and no product formed by 1,2-additon, is to be explained.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 Free radical is an atom, molecule or ion that has an unpaired electron, which makes it highly chemically reactive.
舧 Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
舧 Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
舧 Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
舧 The reaction in which there is addition of hydrogen molecule is called hydrogenation reaction.
舧
舧 Hydrogenation with platinum as a catalyst is used to convert unsaturated carbohydrates to saturated hydrocarbons
舧 Oxidation of
舧 Ozonolysis helps convert the carbon–carbon double bonds to carbon–oxygen double bond (carbonyl compounds).
舧 Dimethyl sulfide is used as a reducing agent that decomposes the intermediate formed into the carbonyl group.
舧 NBS (nitro-bromo succinimide) is a special reagent used for bromination of allylic carbocations.
舧 Bromine replaces the hydrogen attached to the carbon adjacent to the carbon bearing double bond.
舧 This method of using NBS can produce allylic bromides without bromine reacting with the double bond.
舧 Dehydration of a primary alcohol in the presence of a mineral acid like concentrated sulfuric acid results in the formation of alkene via E2 elimination.
舧 The stability of carbocation:
舧 The 1,2 – addition to a diene is the addition of an electrophile to the carbon designated as 1 and a nucleophile to the carbon designated as 2. The positions of carbons as 1 and 2 are not according to the IUPAC numbering of the molecule, but as a conjugated diene molecule. 1,4-addition results in addition of hydrogen to thee carbon designated as 1 and a halogen to the carbon designated as 4.
舧 The mechanism of 1,2 addition and 1,4-addition of hydro halogenation is given below:
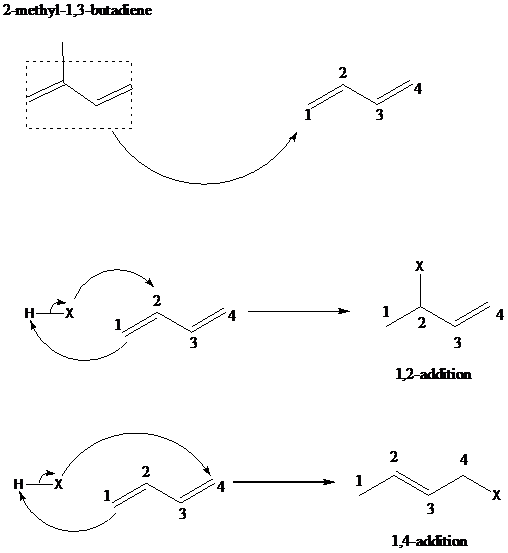
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
- Acid-catalyzed dehydration of 3,3-dimethyl-2-butanol gives three alkenes: 2,3-dimethyl-2-butene, 3,3-dimethyl- 1-butene, and 2,3-dimethyl-1-butene. Draw the structure of the carbocation intermediate leading to the formation of 2,3-dimethyl-2-butene. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • If more than one structure fits the description, draw them all. • Separate structures with + signs from the drop-down menu. / H₂C CH₂ CH3 CH3 ChemDoodleⓇ On []arrow_forwardAcid-catalyzed dehydration of 3,3-dimethyl-2-butanol gives three alkenes: 2,3-dimethyl-2-butene, 3,3-dimethyl-1-butene, and 2,3-dimethyl-1- butene. Draw the structure of the carbocation intermediate leading to the formation of 2,3-dimethyl-2-butene. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. If more than one structure fits the description, draw them all. Separate structures with + signs from the drop-down menu. C P opy aste ChemDoodle >arrow_forwardWhich isomer of 1-bromo-3-isopropylcyclohexane reacts faster when refluxed with potassium tert-butoxide, the cis isomer or the trans isomer? Draw the structure of the expected product from the faster-reacting compound.arrow_forward
- (dehydrobromination) A chemist carried out an elimination reaction of 1,1-dimethyl-2- bromocyclopentane. The chemist expected the reaction to yield alkene Z as product. However, alkene Z DID NOT form. Instead, three alkenes were produced: one alkene was the major product, the other two alkenes was the minor products. What was the major alkene product that formed? Br Select one: A. I B. II C. IV D. III alcohol HEAT || Z = + (HBr) IVarrow_forwardName (including E/Z stereochemistry) the five alkenes that can produce 3-bromo-3-methylhexane on reaction with HBr. Draw the skeletal structure of each molecule.arrow_forwardIdentify two alkenes that react with HBr to form 1-bromo-1-methylcyclohexane without undergoing a carbocation rearrangement.arrow_forward
- Below are the structures for trans-1-chloro-2-t-butylcyclohexane and cis-1- chloro-2-t-butylcyclohexane. Both molecules can react with a base to form an alkene. cis-1-chloro-2-t-butylcyclohexane reacts 10x faster than trans-1-chloro2-t-butylcyclohexane. Use chair conformations to explain why the cis isomer is more reactive.arrow_forwardDraw a product that could be formed when 1,3-butadiene and (Z)-2- butenedial. Include any relative stereochemistry. <¢ Drawing Q Atoms, Bonds and Rings Draw or tap a new bondarrow_forwardOne compound that contributes to the “seashore smell” at beaches in Hawai‘i is dictyopterene D', a component of a brown edible seaweed called limu lipoa. Hydrogenation of dictyopterene D' with excess H2 in the presence of a Pd catalyst forms butylcycloheptane. Ozonolysis with O3 followed by (CH3)2S forms CH2(CHO)2, HCOCH2CH(CHO)2, and CH3CH2CHO. What are possible structures of dictyopterene D'?arrow_forward
- Addition of HBr to allene (CH2=C=CH2) forms 2-bromoprop-1-ene ratherthan 3-bromoprop-1-ene, even though 3-bromoprop-1-ene is formed froman allylic carbocation. Considering the arrangement of orbitals in theallene reactant, explain this result.arrow_forwardChlordane, like DDT, is an alkyl halide that was used as an insecticide for crops such as corn and citrus and for lawns. In 1983, it was banned for all uses except against termites, and in 1988, it was banned for use against termites as well. Chlordane can be synthesized from two reactants in one step. One of the reactants is hexachlorocyclopentadiene. What is the other reactant?arrow_forwardThe two alkenes below react with HI at different rates. CH3CH=CHCH3 and CH₂=CH₂ Draw the structural formula of the MAJOR product formed by the alkene having the HIGHER reaction rate. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. TAYY [ ] در ChemDoodle Ⓡarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





