
Concept explainers
(a)
Interpretation:
The statement “Nonanal has a lower melting point than nonane” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(a)
Answer to Problem 2MCP
The given statement is false because the melting point of nonanal will be higher than nonane due to the presence of strong dipole-dipole attractions in nonanal.
Explanation of Solution
Nonanal is an aldehyde and nonane is an
The carbonyl group of an aldehyde or a ketone is polar because oxygen is more electronegative than carbon. This produces a dipole in which the oxygen carries a partial negative charge and the carbon carries a partial positive charge. Because of the dipole-dipole attractions between molecules are stronger than London dispersion forces,
(b)
Interpretation:
The statement “Nonanal has a higher boiling point than nonanol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(b)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
Nonanal is an aldehyde and nonanol is an alcohol.
Alcohols have strong intermolecular hydrogen bonding. The
Aldehydes have lower boiling points when compared to alcohols, showing the presence of weak intermolecular dipole-dipole forces. Aldehydes and ketones cannot form hydrogen bond with other aldehydes or ketones due to the absence of oxygen-hydrogen bond in the carbonyl group.
Hence, the statement “Nonanal has a higher boiling point than nonanol” is true.
(c)
Interpretation:
The statement “nonanal is not soluble in water” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(c)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
Aldehydes with five or fewer carbon atoms are soluble in water.
Nonanal is a saturated fatty aldehyde with chain length containing nine carbon atoms. The hydrophobic part is non-polar and is insoluble in water. Therefore, the given statement is true.
(d)
Interpretation:
The statement “Nonanal is polar” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(d)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The structure of nonanal is,
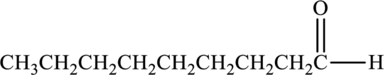
Nonanal consists of carbonyl carbon attached with carbon containing groups. Because of the presence of polar carbonyl group, nonanal is polar in nature. Hence, the given statement is true.
(e)
Interpretation:
The line formula of nonanal has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(e)
Answer to Problem 2MCP
The given line formula of nonanal is false because it represents octanal.
Explanation of Solution
The given line formula is,

The above line formula represents octanal. Therefore, it is false.
The line formula of nonanal is,

(f)
Interpretation:
The statement “Nonanal has weaker dispersion forces between molecules than heptanal” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(f)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The dispersion forces decreases with increase in number of carbon atoms. Nonanal has nine carbon atoms and heptanal has seven carbon atoms, the dispersion forces present in nonanal would be weaker than that of heptanol. Therefore, the given statement is true.
(g)
Interpretation:
The statement “Nonanal is solid at room temperature” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(g)
Answer to Problem 2MCP
The given statement is false because it appears as liquid at room temperature.
Explanation of Solution
Nonanal is liquid that is characterized by a rose-orange odor. It is insoluble in water and is found in twenty essential oils including rose and citrus oils and several species of pine oil.
(h)
Interpretation:
The statement “Oxidation of nonanal would produce a primary alcohol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(h)
Answer to Problem 2MCP
The given statement is false because the oxidation of nonanal would produce a carboxylic acid.
Explanation of Solution
Oxidation of nonanal would produce a carboxylic acid named nonanoic acid. Nonanal is produced by the reduction of primary alcohol (nonanol).
(i)
Interpretation:
The statement “Nonanal is more oxidized than nonanol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(i)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The presence of hydrogen bonding capabilities and the presence of one oxygen atoms along with an extra hydrogen atom makes them more oxidized than nonanol.
Hence, the statement “Nonanal is more oxidized than nonanol” is true.
(j)
Interpretation:
The statement “The reaction of nonanal with an ethanol molecule would produce an acetal” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(j)
Answer to Problem 2MCP
The given statement is false because reaction of nonanal with an ethanol molecule would produce hemiacetal.
Explanation of Solution
A hemiacetal is formed when nonanal reacts with one molecule of ethanol. The given statement is false because acetals are generally produced from two molecules of alcohols with aldehydes/ketones.
(k)
Interpretation:
The statement “The enol structure of nonanal” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(k)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The keto-enol form of nonanal is,
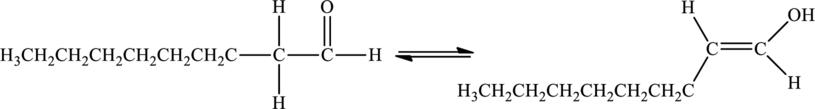
Hence, the given statement is true.
Want to see more full solutions like this?
Chapter 13 Solutions
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





