
Concept explainers
Interpretation:
The compound, which reacts faster with sodium methoxide in methanol in each of the given pair of compounds is to be determined and the chemical equation for the faster reaction is to be written.
Concept introduction:
Nucleophilic
In nucleophilic aromatic substitution reactions, the nucleophile substitutes a leaving group from the aryl ring.
Aryl halides bearing an electron withdrawing substituent undergo nucleophilic substitution rapidly.
The substituents attached ortho and para with respect to the halogen atom in the aryl halide react at similar rates. The substituents attached at meta position in the aryl halide react at slower rates than ortho and para substituents.
Electron withdrawing substituents stabilize the intermediate carbanion formed and thus are strongly activating substituents in the nucleophilic aromatic substitution reactions.
Electron donating substituents destabilize the intermediate carbanion formed and thus are strongly deactivating substituents in the nucleophilic aromatic substitution reactions.
Answer to Problem 43P
Solution:
a)
The reaction is as follows:
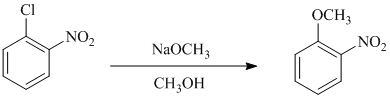
b) In between
The reaction is as follows:

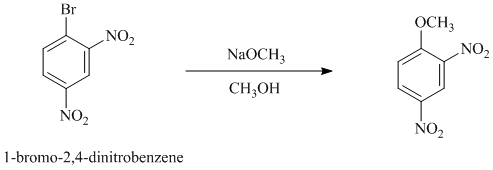
c) In between
The reaction is as follows:
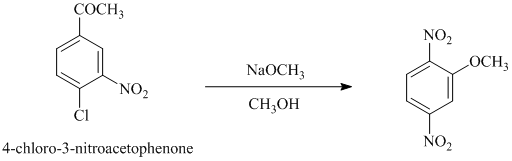
d) In between
The reaction is as follows:
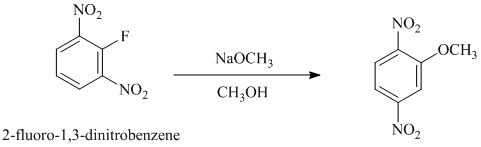
e) In between
The reaction is as follows:
Explanation of Solution
a)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
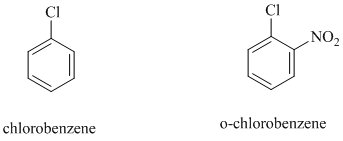
In chlorobenzene, a chlorine atom is attached to the benzene ring while in
The reaction of
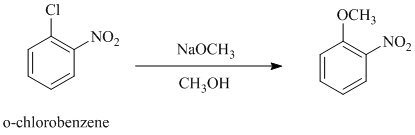
b)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
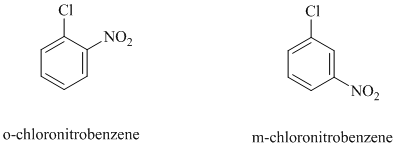
In both the given aryl halides, a strong electron withdrawing substituent is attached on the ring. In
The reaction of
c)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
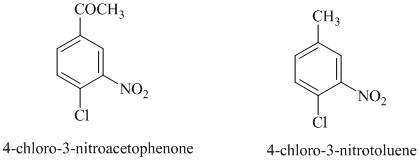
In
In
Thus, in between
The reaction of

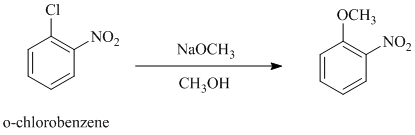
d)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
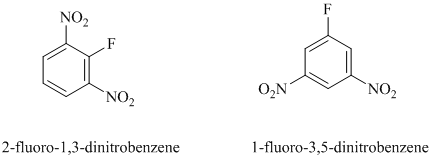
Nitro substituents are strong electron withdrawing substituents.
In
Electron withdrawing substituents at ortho and para positions activate the ring more than the electron withdrawing substituents at meta positions.
Thus, in between
The reaction of

e)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
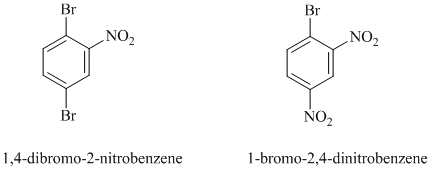
In
The reaction of

Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardDraw structural formulas for (1) the alkyltriphenylphosphonium salt formed by treatment of each haloalkane with triphenylphosphine, (2) the phosphonium ylide formed by treatment of each phosphonium salt with butyllithium, and (3) the alkene formed by treatment of each phosphonium ylide with acetone.arrow_forwardIdentify the compound in each of the following pairs that reacts more rapidly in SN2 reactions: (a) 1-bromopentane or 3-bromopentane (b) 2-chloropentane or 2-fluoropentane (c) 2-bromopropane or 1-bromohexane (d) 1-chlorohexane or cyclohexyl chloridearrow_forward
- Which of the following is a substrate product when benzene is first treated with HNO3 and H2SO4, and THEN treated (in a second step) with Br2 and FeCl3 (or FeBr3)? 1-bromo-3-nitrobenzene 1-chloro-1-nitrobenzene 1-bromo-1-nitrobenzene aniline cis-1-bromo-2-nitrocyclohexanearrow_forwardRank the following compounds from greatest tendency to least tendency to undergo nucleophilic aromatic substitution: chlorobenzene 1-chloro-2,4-dinitrobenzene p-chloronitrobenzenearrow_forwardMarkovnikov’s Rule is required in order to predict that the major substrate product in the reaction between HCl (hydrochloric acid) and ___ will be ___ . benzene; chlorobenzene 1-butene; 1-chlorobutane 1-hexene; 2-chlorohexane 3 of these 4 responses are correct 1-octene; 2-bromooctanearrow_forward
- Write reactions of aniline with the following reagents: a. CH3COCl b. Br2 c. CH3Iarrow_forwardSyntheses of each of the following compounds have been reported in the chemical literature. Using the indicated starting material and any necessary organic or inorganic reagents, describe short sequences of reactions that would be appropriate for each transformation. (a) 1,1,5-Trimethylcyclononane from 5,5-dimethylcyclononanonearrow_forwardRank the following compounds from greatest tendency to least tendency to undergo nucleophilic aromatic substitution: chlorobenzene 1-chloro-2,4-dinitrobenzene p-chloronitrobenzene a. Rank the same compounds from greatest tendency to least tendency to undergo electrophilic aromatic substitution.arrow_forward
- Which of the following sets of reagents would not be an acceptable method for the preparation of ethylacetate? A. acetic acid, ethanol, and an acid catalyst B. Sodium acetate and ethanol C. Acetic anhydride and ethanol D. Sodium acetate and ethyliodidearrow_forwardA reaction flask contains 2-bromopentane in an ethanolic solution of sodium ethoxide at room temperature and result in the formation of two olefnic products. What is responsible for the formation of major and minor products. A. Different activated complex involved in the mechanism. B. Bimolecular nucleophilic substitution reaction. C. Bimolecular elimination reaction D. The presence of sodium ethoxide. E. The hybridization nature of secondary carbocationarrow_forwardGive the products, if any, of each of the following reactions: a. benzonitrile + methyl chloride + AlCl3 b. phenol + Br2 c. benzoic acid + CH3CH2Cl + AlCl3 d. benzene + 2 CH3Cl + AlCl3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
