
Concept explainers
Draw structural formulas for (1) the alkyltriphenylphosphonium salt formed by treatment of each
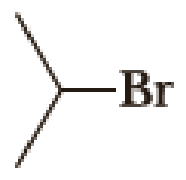
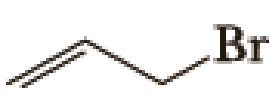
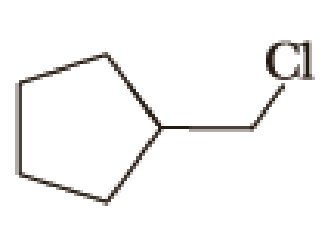
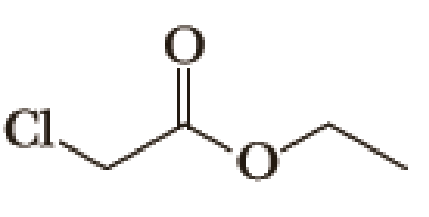

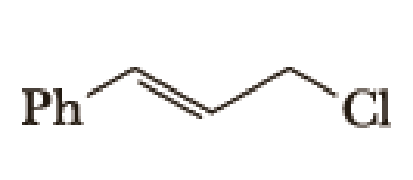
(a)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
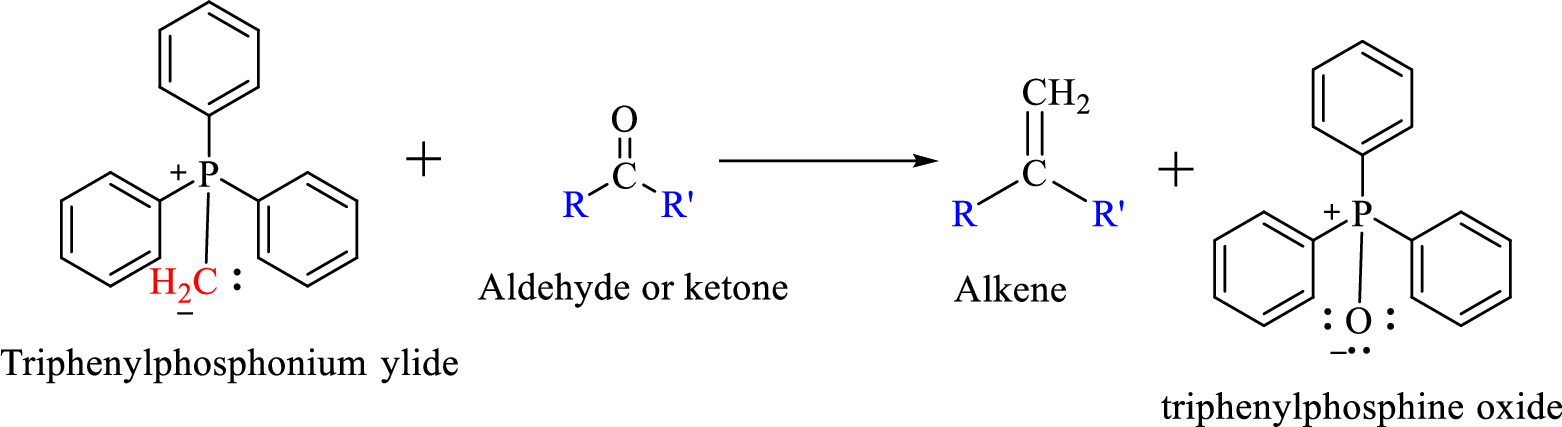
Mechanism:
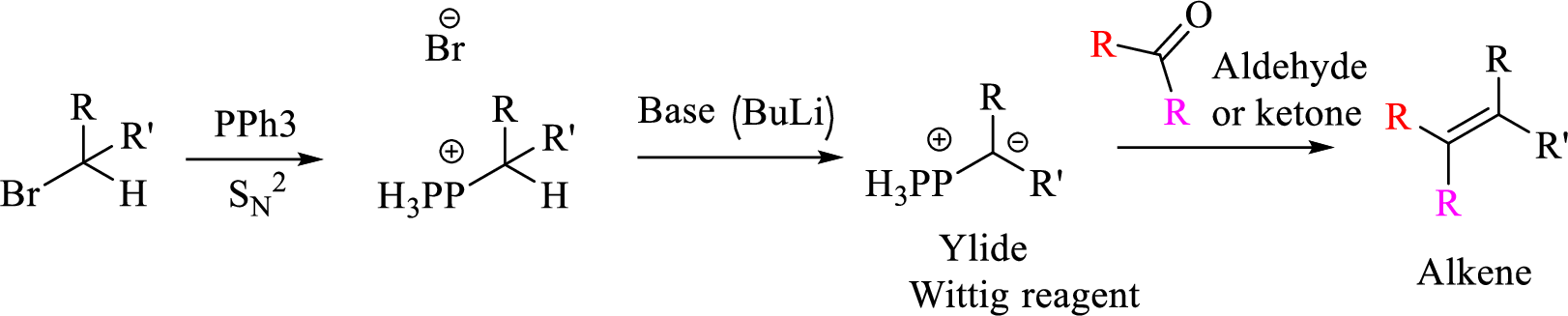
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
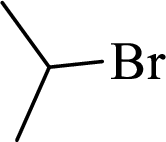
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
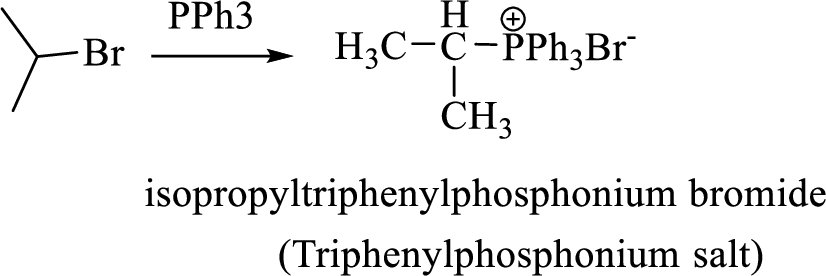
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
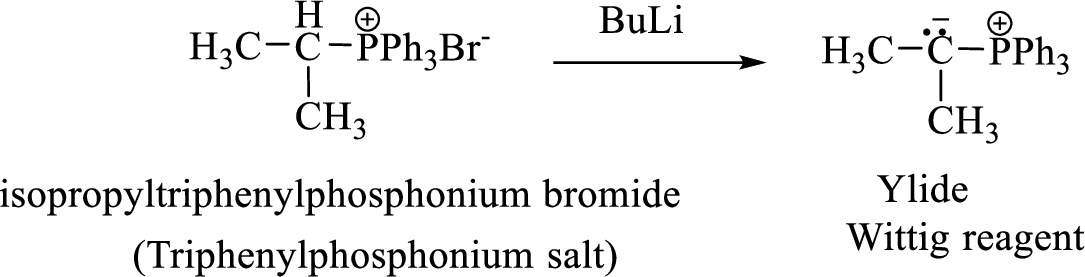
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
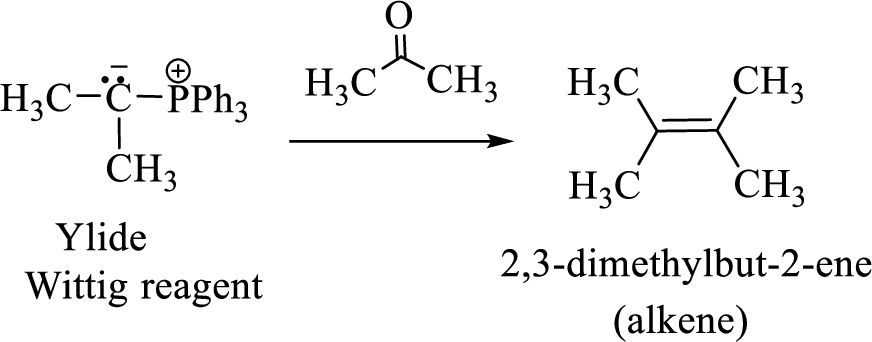
(b)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
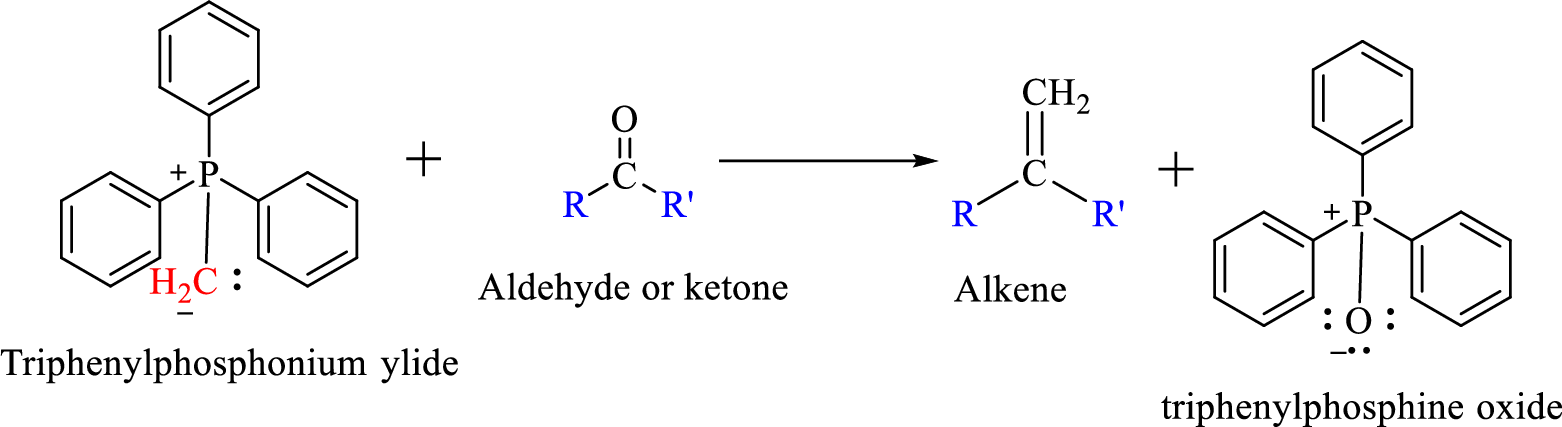
Mechanism:
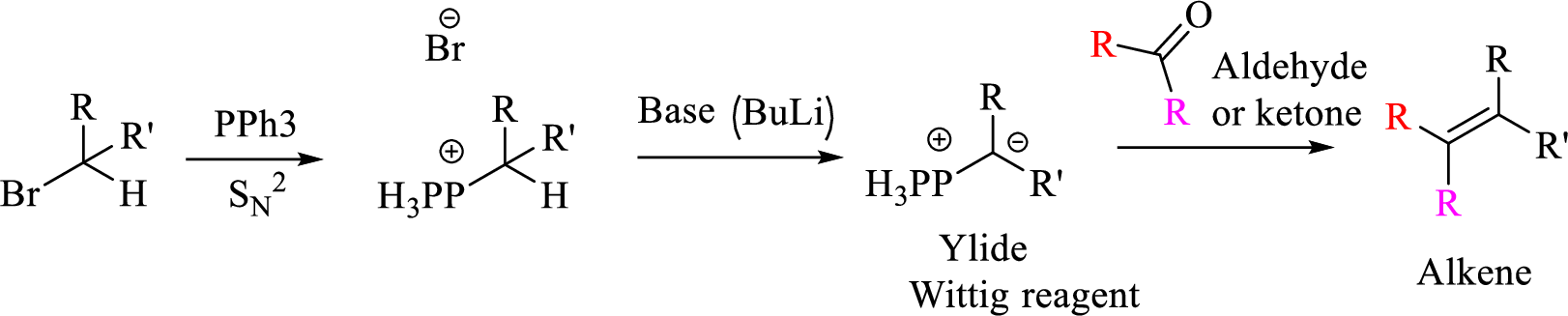
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
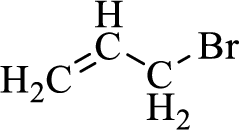
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
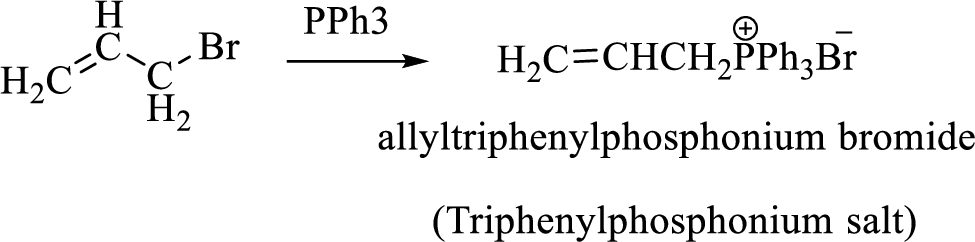
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
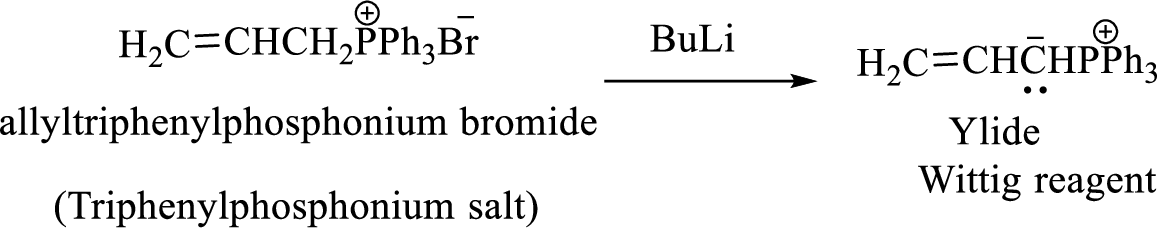
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
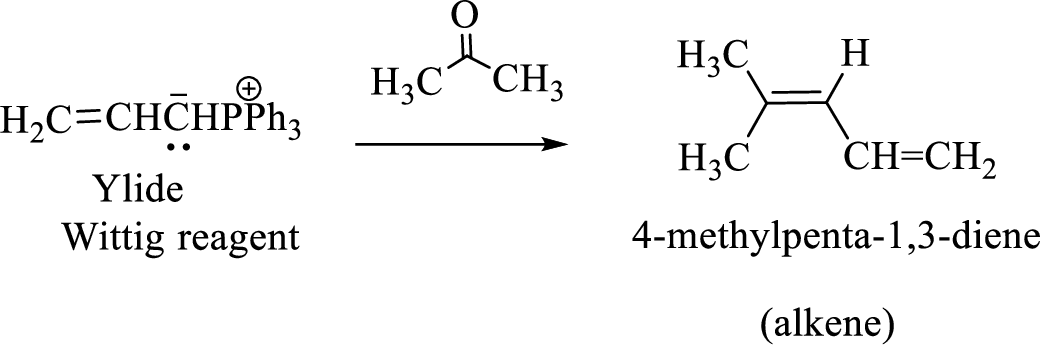
(c)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
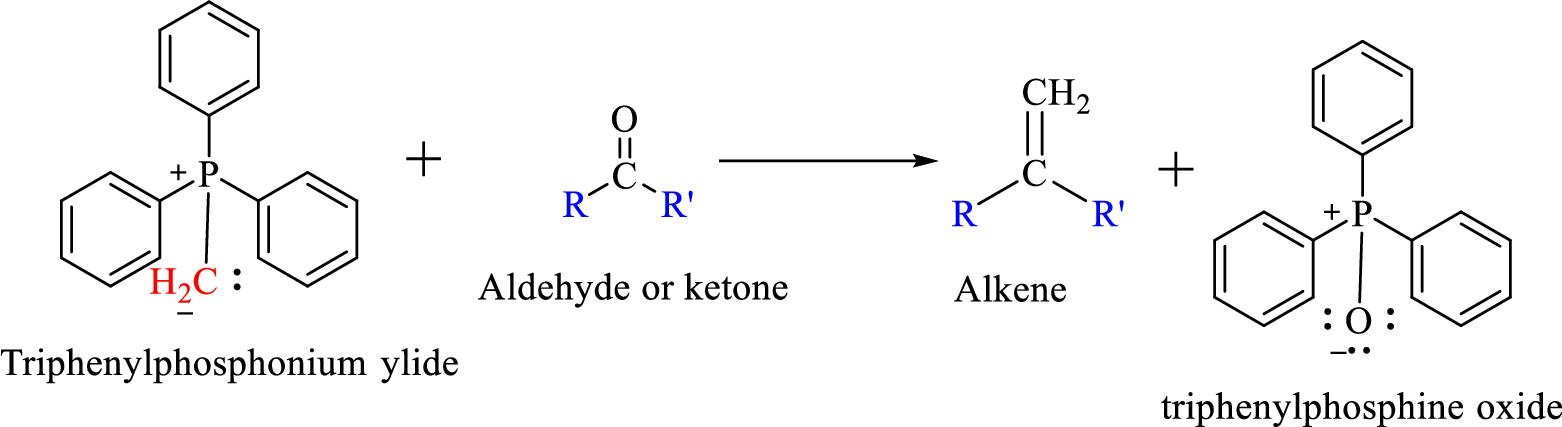
Mechanism:
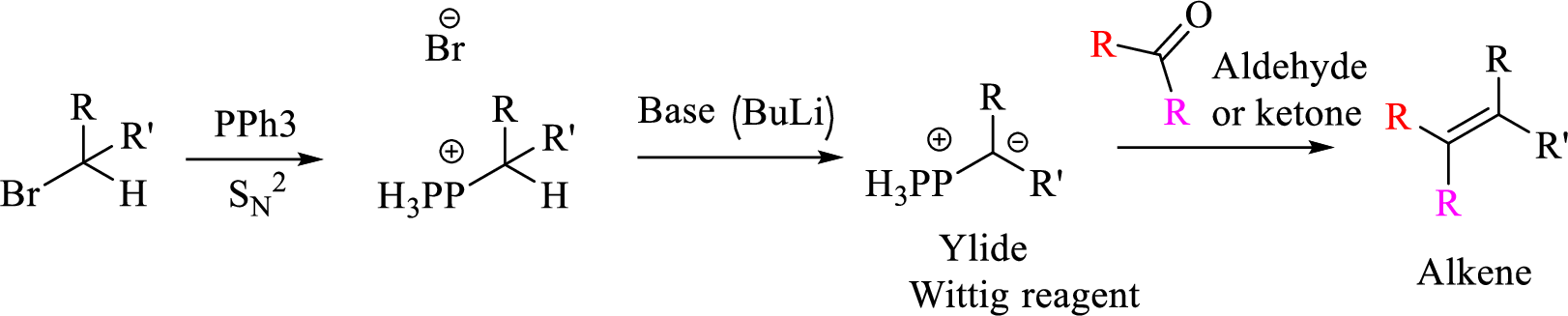
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
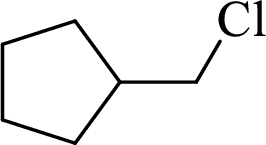
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
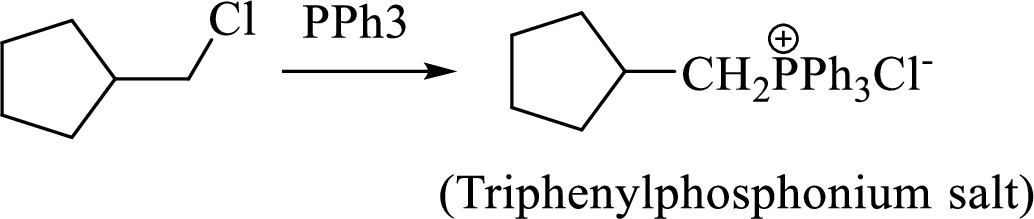
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
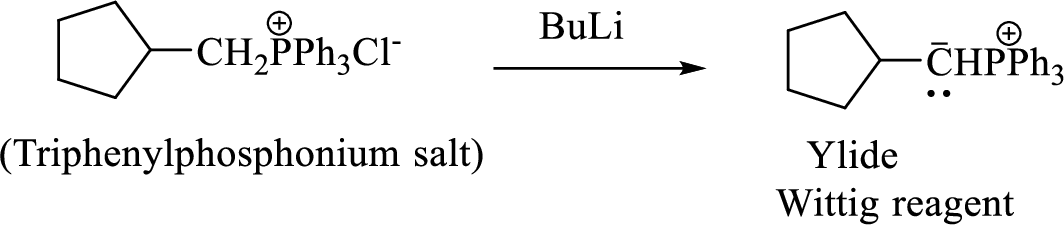
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
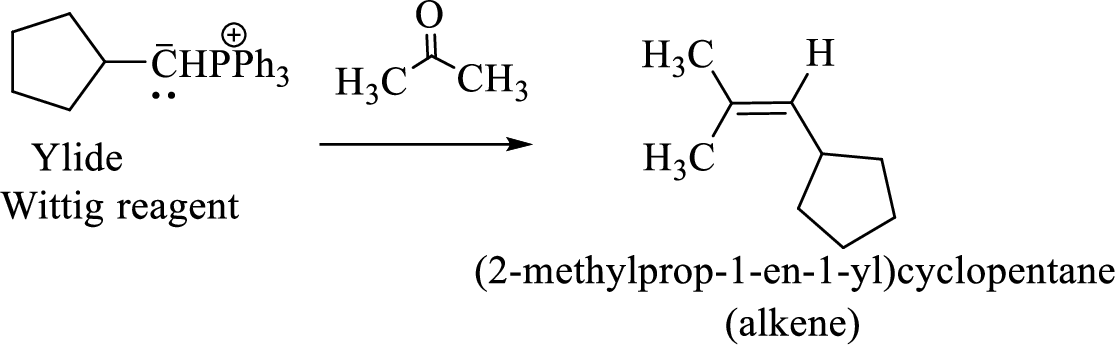
(d)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
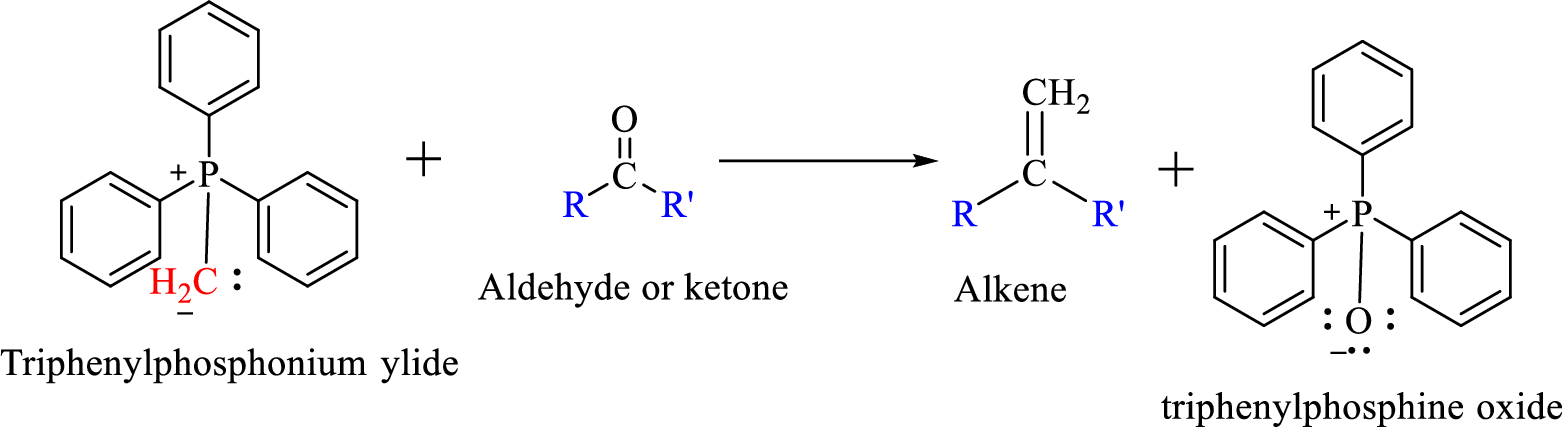
Mechanism:
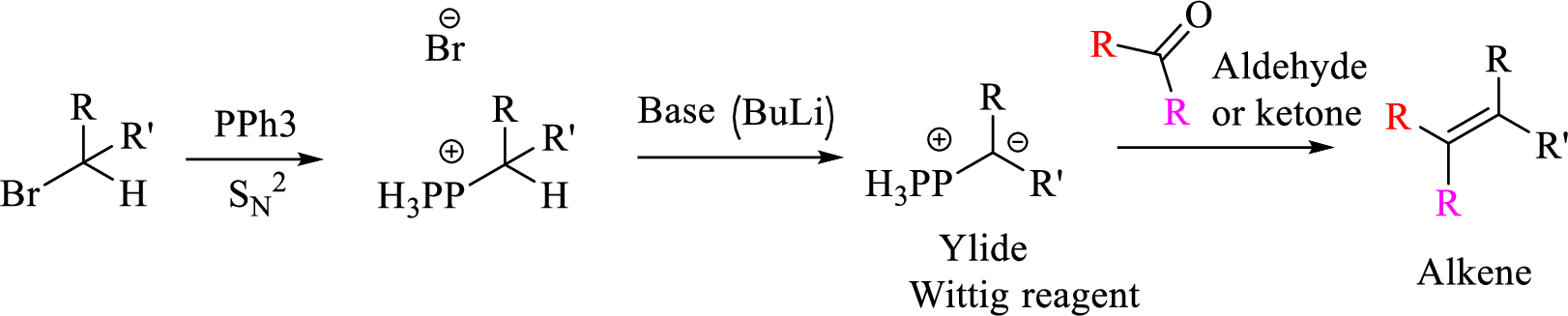
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
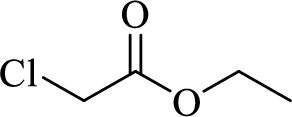
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
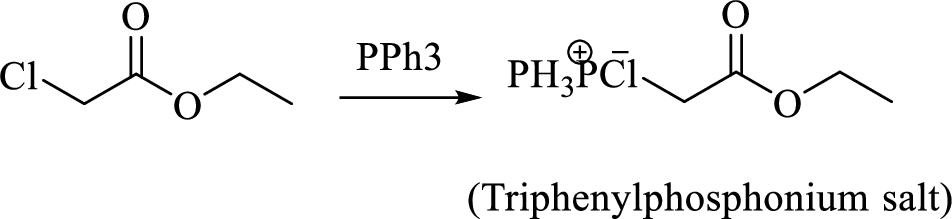
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
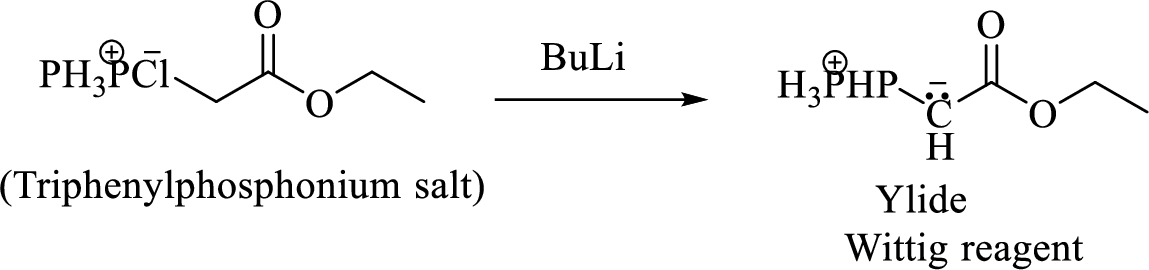
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
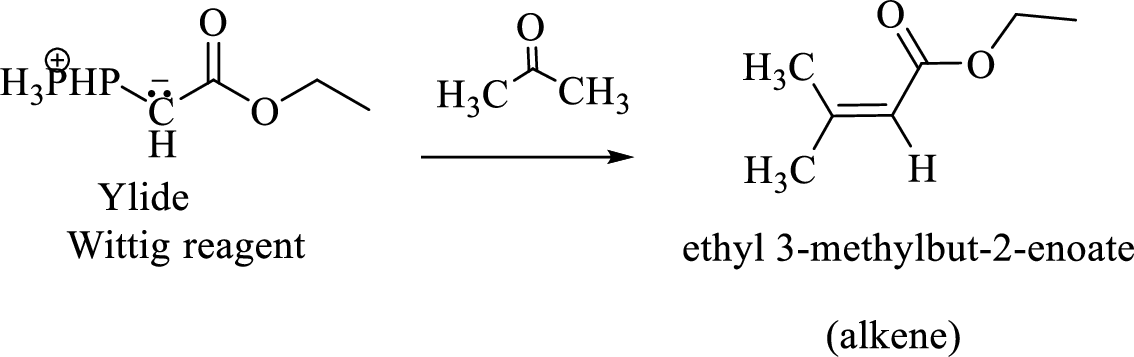
(e)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
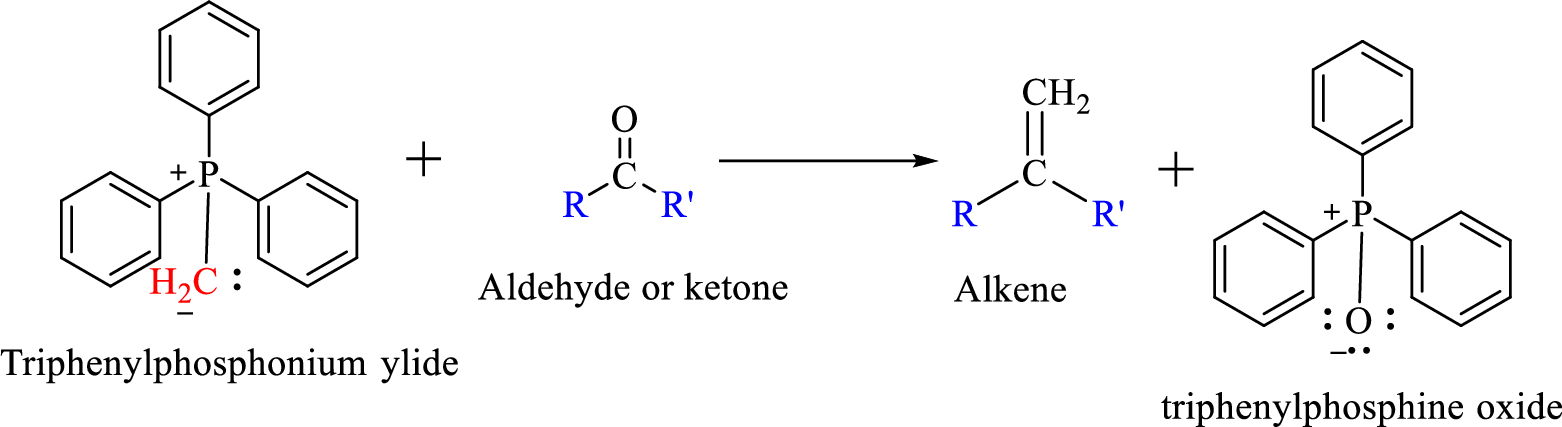
Mechanism:
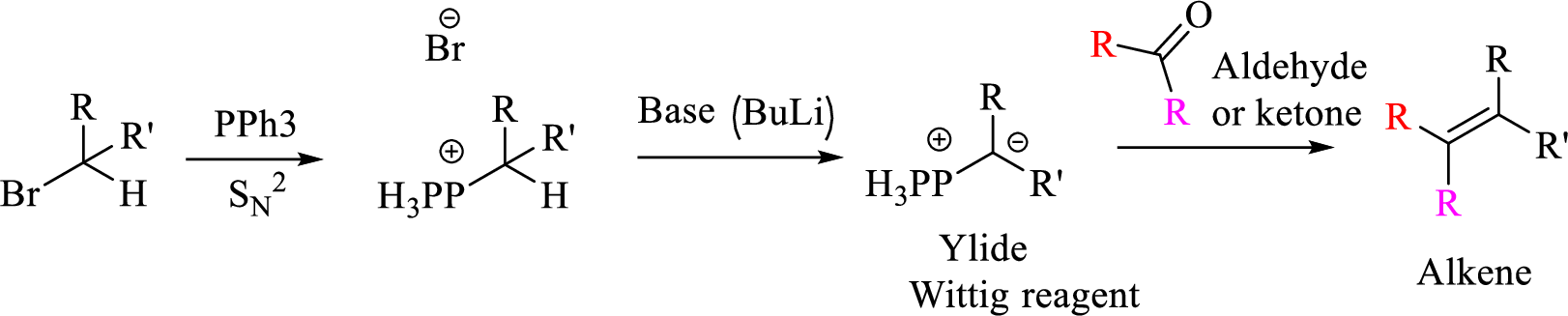
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.

When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
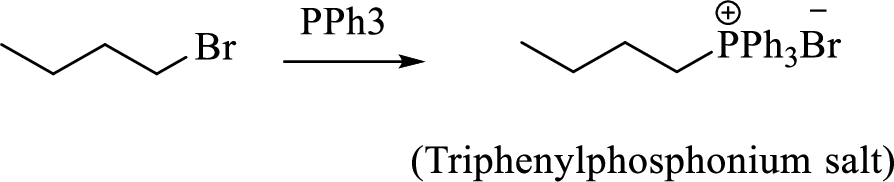
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
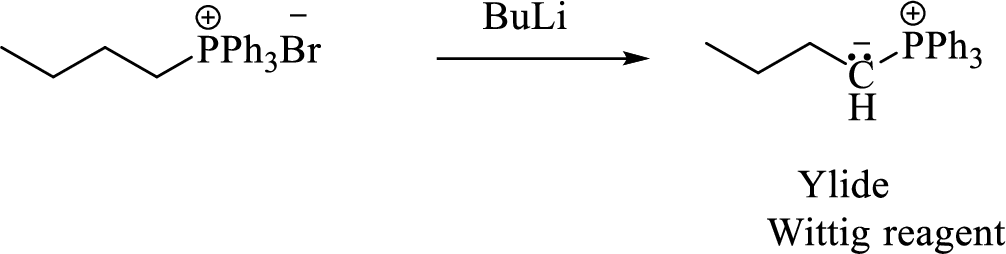
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
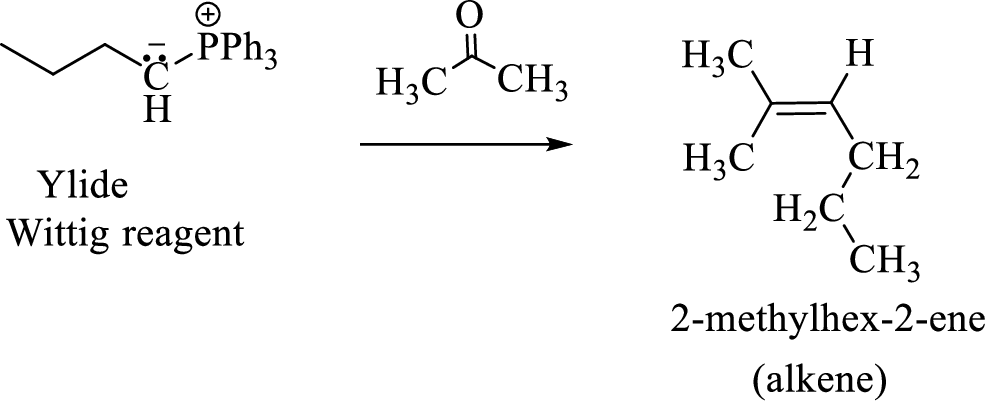
(f)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
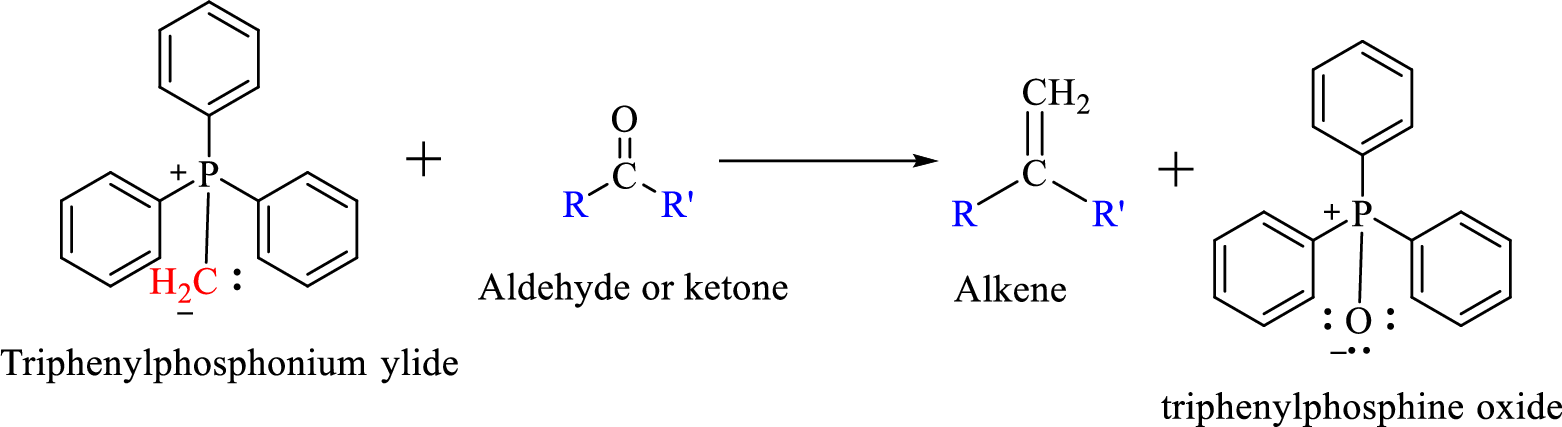
Mechanism:
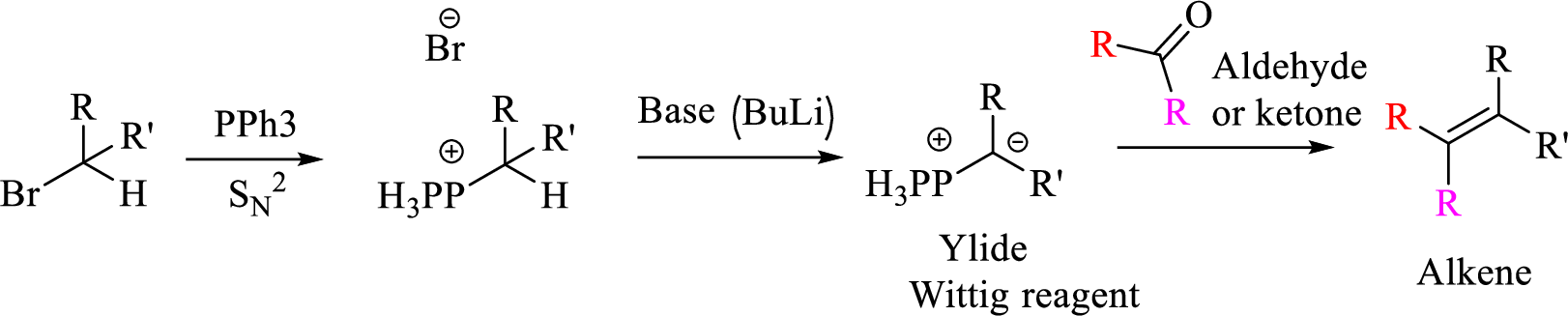
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.

When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
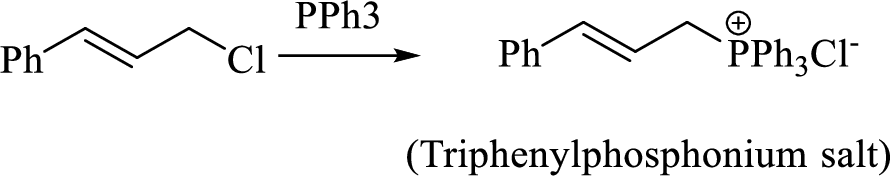
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
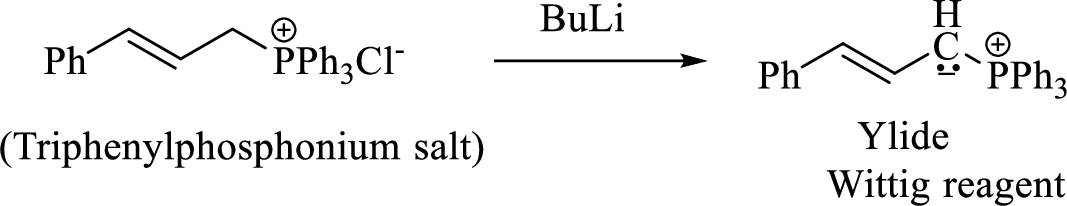
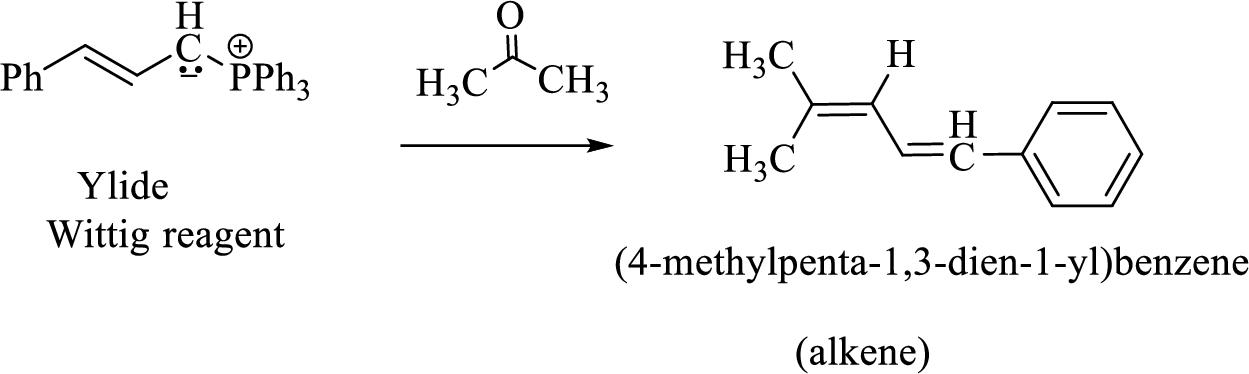
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Thermodynamics, Statistical Thermodynamics, & Kinetics
Essential Organic Chemistry (3rd Edition)
Chemistry: The Molecular Nature of Matter
Chemistry: A Molecular Approach (4th Edition)
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardEarly organic chemists used the Hofmann elimination reaction as the last step of a process known as a Hofmann degradation—a method used to identify amines. In a Hofmann degradation, an amine is methylated with excess methyl iodide in a basic solution, treated with silver oxide to convert the quaternary ammonium iodide to a quaternary ammonium hydroxide, and then heated to allow it to undergo a Hofmann elimination. Once the alkene product is identified, working backward gives the structure of the amine. Identify the amine in each of the following cases: a. 4-Methyl-2-pentene is obtained from the Hofmann degradation of a primary amine. b. 3-Methyl-1-butene is obtained from the Hofmann degradation of a primary amine. c. 2-Methyl-1-3-butadiene is obtained from two successive Hofmann degradations of a secondary amine.arrow_forwardStarting with benzene and using any other necessary reagents of your choice, what are the possible syntheses for the following compound?arrow_forward
- . Write short notes on the following, mentioning reagents, reaction conditions, products formed, mechanism, purification and precautions where necessary• Hydrolysis of 2-bromo-2-methylbutane• Nitration of benzene• Reduction of Nitrobenzene• Ammonolysis of benzoylchloride• Ozonolysis of 2-methylbut-2-enearrow_forwardSynthesize the following compound using as carbon sources only benzene, toluene, benzaldehyde, acetic anhydride, and alcohols of four carbons or fewer. You can use any inorganic reagents and triphenyl phosphinearrow_forwardSuggest how you would synthesize each compound, use cyclopentanone as one of the reagentsarrow_forward
- How would you perform an experiment to produce cyclohexanol from cyclohexene? Describe in detail the reagents and glassware you would use including approximate amounts and concentrations of reagents. What steps would be needed to purify the cyclohexanol from the other reagents used in the reaction?arrow_forwardThe following compound may be synthesized through alkylation of an appropriate enamine with an alkyl bromide, followed by hydrolysis of the resulting immonium ion. Using this strategy, provide the necessary starting materials for the synthesis.arrow_forwardThe following molecule can undergo keto–enol tautomerization upon addition of hydroxide. Draw the structure of the enol form and the mechanism by which it forms.arrow_forward
- Draw the principal organic product for the reaction of 2-bromobutane with magnesium in diethyl ether, followed with benzaldehyde in diethyl ether, and then followed by dilute acid...arrow_forwardThe following questions pertain to the esters shown and behavior under conditions of the Claisen condensation.(a) Two of these esters are converted to β-keto esters in good yield on treatment with sodium ethoxide and subsequent acidification of the reaction mixture. Which two are these? Write the structure of the Claisen condensation product of each one. (b) One ester is capable of being converted to a β-keto ester on treatment with sodium ethoxide, but the amount of β-keto ester that can be isolated after acidification of the reaction mixture is quite small. Which ester is this? (c) One ester is incapable of reaction under conditions of the Claisen condensation. Which one? Why?arrow_forwardA hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
