
(a)
Interpretation:
The statement “
(a)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
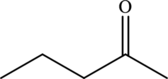
Hence, the statement
(b)
Interpretation:
The statement “
(b)
Answer to Problem 4MCP
The given statement is false because
Explanation of Solution
Alcohols have strong intermolecular hydrogen bonding. The
Ketones have lower boiling points when compared to alcohols, representing the presence of weak intermolecular dipole-dipole forces. Due to weaker intermolecular hydrogen and weaker dipole-dipole attractions in ketones, they have lower boiling points compared to alcohols.
Hence, the given statement is false because
(c)
Interpretation:
The statement “
(c)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
(d)
Interpretation:
The statement “
(d)
Answer to Problem 4MCP
The given statement is false because
Explanation of Solution
The structure of
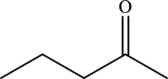
The carbonyl group present in
The statement “
(e)
Interpretation:
The statement “
(e)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
The structure of
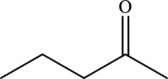
From the structure of
The statement “
(f)
Interpretation:
The statement “
(f)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
The presence of polar carbonyl group and the attractive forces between the carbonyl-containing compounds include London dispersion forces between the hydrocarbon chains and dipole-dipole attractions between the carbonyl groups. Because the dipole-dipole attractions between the molecules are stronger than London dispersion forces,
(g)
Interpretation:
The statement “
(g)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
(h)
Interpretation:
The statement “
(h)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Ketones cannot form hydrogen bonds with each other, but they form intermolecular hydrogen bonds with water. As a result, the smaller members of ketones (five or fewer carbon atoms) are soluble in water. Hence, the statement “
(i)
Interpretation:
The statement “
(i)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Ketone is carbonyl compounds. The carbonyl group of the ketone is polar and can participate in dipole-dipole attractions.
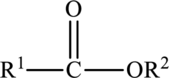
The structure of
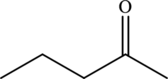
(j)
Interpretation:
The statement “
(j)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of
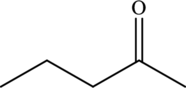
The molecular formula of pentanal is

Both these are structural isomers with each other and hence, the statement “
Want to see more full solutions like this?
Chapter 13 Solutions
GEN ORGANIC CHM LL W/CONNECT
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





