
Concept explainers
What alkyd halide is formed when each

b.
c.
(a)
Interpretation:
The resulting alkyl halide should be identified after reacting following alkene with

Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond that has the general formula of
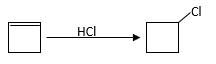
Reaction of an alkene with
Hydro halogenation reaction of alkenes follows Markovnikov's rule.
Answer to Problem 55P
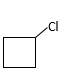
Explanation of Solution
Unsaturated (
Hydro halogenation reaction of alkenes follows Markovnikov's rule. When the addition of
Refer to the below reaction;
(b)
Interpretation:
The resulting alkyl halide should be identified after reacting following alkene with
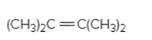
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydro halogenation reaction of alkenes follows Markovnikov's rule.
Answer to Problem 55P
Explanation of Solution
Unsaturated (
Hydro halogenation reaction of alkenes follows the Markovnikov's rule. When addition of
Refer to the below reaction;
(c)
Interpretation:
The resulting alkyl halide should be identified after reacting following alkene with
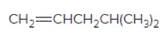
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydro halogenation reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 55P
Explanation of Solution
Unsaturated (
Hydro halogenation reaction of alkenes follows the Markovnikov's rule. When the addition of
Refer to the below reaction;
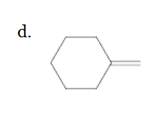
(d)
Interpretation:
The resulting alkyl halide should be identified after reacting following alkene with
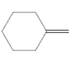
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydro halogenation reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 55P
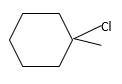
Explanation of Solution
Unsaturated (
Hydro halogenation reaction of alkenes follows the Markovnikov's rule. When
Refer to the below reaction;
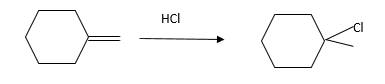
Want to see more full solutions like this?
Chapter 13 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
- How does hybridization of the substituted carbon atom change when an alcohol is converted into an aldehyde? An aldehyde to a carboxylic acid?arrow_forward6. Draw the correct structures for the following: a. what is the correct structure of 2-heptene? b. what is the correct structure of 4-octanone? c. what is the correct structure of dipropyl ether? d. what is the correct structure of 3-ethyl hexane? e. what is the correct structure of 2,2 dimethyl 1-hexanol?arrow_forwardDraw propyne and fill in all H’s .arrow_forward
- 2. a. What is the chemical structure of naphthalene, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? _________________________arrow_forwarda) Write the name b) draw the structure product formed from the reaction of 3-methylpent-2-ene with [1] 9-BBN; [2] H2O2, OH-arrow_forwardDraw all the alkyl halides with molecular formula C 5H 11Cl formed when pentane (CH 3CH 2CH 2CH 2CH 3) is heated with Cl 2.arrow_forward
- CH3-CH2-OH reacts with H+/140C = ? + H2Oarrow_forwardhi! i need answers on letters d to f. The instructions says give the IUPAC name of each compound. Thank u!arrow_forwardWhat alkenes are formed when each alcohol is treated with H 2SO 4? Use the Zaitsev rule to predict the major product.arrow_forward
- Dexamethasone is a halogen-containing steroid used to treat infl ammation in rheumatoid arthritis and other conditions. (a) Classify the alkyl halide in dexamethasone as 1 °, 2 °, or 3 °. (b) Classify the hydroxyl groups as 1 °, 2 °, or 3 °.arrow_forward1. Draw the structure for each compound. a.(3R)-3-methylhexane b. (3R,5S,6R)-5-ethyl-3,6-dimethylnonanearrow_forwarddraw 2-cyclohexene-1-olarrow_forward

 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





