
Concept explainers
Interpretation:
The Lewis diagrams for
Concept Introduction:
The Lewis structure shows the connectivity between atoms by identifying the lone pairs of electrons in a compound. Lewis structures are also known as Lewis dot structures. The valence electrons around an atom are shown by dots. Bonds between atoms are shown by lines and the lone pair of electrons is shown by a pair of dots.
Answer to Problem 77E
The Lewis diagrams for
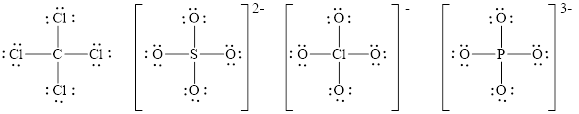
The number of valence electrons present in
The Lewis diagrams for
Explanation of Solution
The number of valence electrons present in
The Lewis diagrams for

Figure 1
The two things that are alike about Lewis diagram for
• The number of valence electrons present in all species (
• The number of atoms attached to central metal atom in all species (
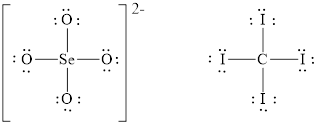
The Lewis diagrams for
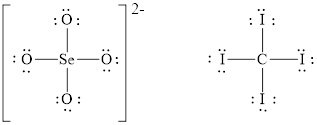
Figure 2
The Lewis diagrams for
The number of valence electrons present in
The Lewis diagrams for
Want to see more full solutions like this?
Chapter 13 Solutions
Introductory Chemistry: An Active Learning Approach
- Write the Lewis structure of NH2OH.arrow_forwardUsing Lewis structures and VSEPR diagrams, describe the shapes (including bond angles and molecular shape names) of AsF3 and AsF5. Based on these shapes, explain why the melting point of AsF3 is higher than that of AsF5.arrow_forwardDraw a Lewis structure for BrCl5 . What is the molecular geometry around the central atom in the Lewis structure?arrow_forward
- Consider peroxynitrite (chemical formula: ONOO−), a structural isomer of the nitrate anion. It is generated in the cell when nitric oxide (NO) reacts with the superoxide radical anion (O2-.). Peroxynitrite is a potent oxidant and nitrating agent and can lead to DNA and protein damage (this is the complete question) Draw the correct Lewis structure for peroxynitrite and indicate approximate bond angles. Tell how many molecular degrees of freedom of motion are present in peroxynitrite and what motions they correspond to.arrow_forwardThe carbonate anion, CO32- , is a resonance hybrid. Draw all of the important resonance structures for this molecule. If an atom has a nonzero formal charge, be sure the formal charge is shown clearly in the structure. Use the resonance structures to calculate the average formal charge on each O atom (which are all equivalent in the "true" structure). [Note: all of the important contributing resonance structures have octets around each atom that desires an octet.]arrow_forwardChloral, Cl3C—CH=O, reacts with water to form the sedative and hypnotic agent chloral hydrate, Cl3C—CH(OH)2. Draw Lewis structures for these substances, and describe the change in molecular shape, if any, that occurs around each of the carbon atoms during the reaction.arrow_forward
- Draw the lewis structure of Cl3C-CH(OH)2.arrow_forwardSulfur tetrafluoride reacts slowly with oxygen gas to form sulfur tetrafluoride monoxide. Write a balanced chemical equation for the reaction. In the sulfur tetrafluoride monoxide molecule the O atom and the four F atoms are bonded to a central S atom. Write a Lewis structure for this molecule in which the formal charges of all atoms is zero. Use bond energies to estimate the enthalpy of the above reaction. (S-F bond energy = 327 kJ/mole). Is it endothermic or exothermic? Determine the electron geometry of the molecule and predict two possible molecular geometries. Which of the two molecular geometries in part d is more likely to be observed? Explain.arrow_forward10.) The structural formula of a certain aldehyde (related to formaldehyde) is H3C-CH2-CHO. Draw a Lewis structure for this aldehyde and determine the number of bonds present. Note that a single or a double or a triple bond counts as one bond. Write the number, not the word.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





