
Concept explainers
How many absorptions would you expect to observe in the 13C NMR spectra of the following compounds?
(a) 1,1-Dimethylcyclohexane
(b) CH3CH2OCH3
(c) tert-Butylcyclohexane
(d) 3-Methyl-l-pentyne
(e) cis-1,2-Dimethylcyclohexane
(f) Cyclohexanone
a)
Interpretation:
The structure of an given molecular formula C8H16 to be predicted using 13CNMR spectra.
Concept introduction:
The 13CNMR spectrum gives information on the different electronic environments of carbon. As like 1HNMR, the number of signals generated in 13CNMR are predicted by performing symmetry operations (rotation or reflection symmetry). Only chemical shift values are reported in the spectrum but not the multiplicity and integration values because the coupling between two neighboring 13C - 13C nuclei are weakly involved due to the low abundance of 13C isotopes of carbon atom.
Broadband-decoupled 13CNMR spectrum: The spectra provide information regarding the total number of carbon environments.
DEPT (Distortionless enhancement by polarization transfer):
i) DEPT -90: The spectrum exhibits signal only from CH group and no signals from CH3, CH2, CH and quaternary carbon (carbon with no protons).
ii) DEPT -135: The spectrum exhibits CH3 groups and CH groups as positive signals (pointing up); CH2 groups appear as negative signals (pointing down) and quaternary carbon does not appear.
The signals appear in each type of spectrum:
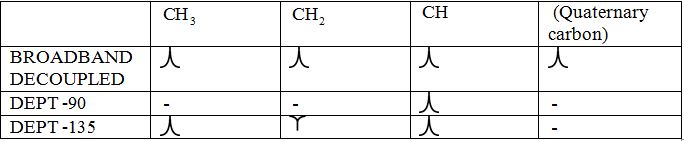
To identify:
The structure of given molecular formula C8H16.
Answer to Problem 47AP
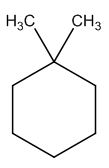
Explanation of Solution
Calculate HDI value:
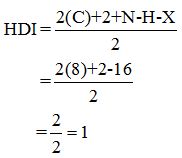
The HDI calculation led to confirm about the presence of a double bond or a ring.
Interpret the given 13CNMR spectrum:
Broadband - decoupled spectrum:
The spectrum shows five signals whereas the given molecular formula also has eight carbon atoms. Thus all the eight carbons have chemically different electronic environments showing signals.
i) The five signals in the region of 0-50ppm indicate the sp3 hybridized carbon atoms which can be methyl / methylene or Methine groups.
DEPT -90: Spectrum has no signals led to confirmation of no CH group in the structure.
DEPT -135 (gives signals of CH2, CH3 and C groups):
ii) The two positive signals indicate the presence of two methyl groups as no signals appear in the DEPT -90 spectrum.
iii) The three negative signals indicate the presence of four methylene groups; only methylene groups appear negative in the spectrum.
iv) So far the group of fragments obtained is
Two -CH3, -C-, four -CH2
From the fragments (CH3 + CH3 + (CH2)5 + C = 16 protons) sixteen protons are obtained whereas the total number of protons from the molecular formula is also sixteen.
The possible structures are:
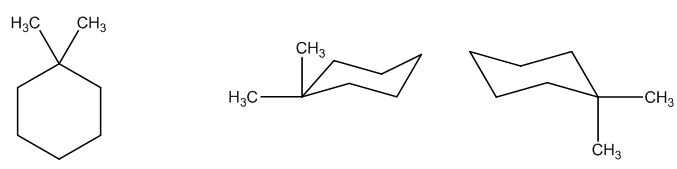
The first structure possesses rotational symmetry and exhibits fewer signals in the spectrum and gets cancelled out.

The structure of a given molecular formula C8H16 is predicted using 13CNMR spectra.
b)
Interpretation:
The structure of an given molecular formula C8H16 to be predicted using 13CNMR spectra.
Concept introduction:
The 13CNMR spectrum gives information on the different electronic environments of carbon. As like 1HNMR, the number of signals generated in 13CNMR are predicted by performing symmetry operations (rotation or reflection symmetry). Only chemical shift values are reported in the spectrum but not the multiplicity and integration values because the coupling between two neighboring 13C - 13C nuclei are weakly involved due to the low abundance of 13C isotopes of carbon atom.
Broadband-decoupled 13CNMR spectrum: The spectra provide information regarding the total number of carbon environments.
DEPT (Distortionless enhancement by polarization transfer):
i) DEPT -90: The spectrum exhibits signal only from CH group and no signals from CH3, CH2, CH and quaternary carbon (carbon with no protons).
ii) DEPT -135: The spectrum exhibits CH3 groups and CH groups as positive signals (pointing up); CH2 groups appear as negative signals (pointing down) and quaternary carbon does not appear.
The signals appear in each type of spectrum:

To identify:
The structure of given molecular formula C3H8O.
Answer to Problem 47AP
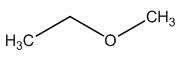
Explanation of Solution
Calculate HDI:
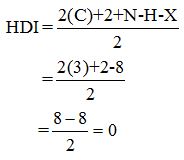
The HDI calculation led to confirm about the absence of a double bond or a ring.
Interpret the given 13CNMR spectrum:
Broadband - decoupled spectrum:
The spectrum shows three signals whereas the given molecular formula also has three carbon atoms. Thus all the three carbons have chemically different electronic environments showing signals.
i) The five signals in the region of 0-50ppm indicate the sp3 hybridized carbon atoms which can be methyl / methylene groups.
DEPT -90: Spectrum has no signals led to confirmation of no CH group in the structure.
DEPT -135 (gives signals of CH2, CH3 groups):
ii) The two positive signals indicate the presence of two methyl groups as no signals appear in the DEPT -90 spectrum.
iii) The spectrum shows three signals whereas the given molecular formula also has three carbon atoms. Thus all the three carbons have chemically different electronic environments showing signals.
iv) So far the group of fragments obtained is
Two -CH3, -CH2
From the fragments (CH3 + CH3 + CH2 = 8 protons) eight protons are obtained whereas the total number of protons from the molecular formula is also eight.
The structure of a given molecular formula C3H8O is predicted using 13CNMR spectra.
c)
Interpretation:
The structure of an given molecular formula C8H16 to be predicted using 13CNMR spectra.
Concept introduction:
The 13CNMR spectrum gives information on the different electronic environments of carbon. As like 1HNMR, the number of signals generated in 13CNMR are predicted by performing symmetry operations (rotation or reflection symmetry). Only chemical shift values are reported in the spectrum but not the multiplicity and integration values because the coupling between two neighboring 13C - 13C nuclei are weakly involved due to the low abundance of 13C isotopes of carbon atom.
Broadband-decoupled 13CNMR spectrum: The spectra provide information regarding the total number of carbon environments.
DEPT (Distortionless enhancement by polarization transfer):
i) DEPT -90: The spectrum exhibits signal only from CH group and no signals from CH3, CH2, CH and quaternary carbon (carbon with no protons).
ii) DEPT -135: The spectrum exhibits CH3 groups and CH groups as positive signals (pointing up); CH2 groups appear as negative signals (pointing down) and quaternary carbon does not appear.
The signals appear in each type of spectrum:

To identify:
The structure of given molecular formula C10H20.
Answer to Problem 47AP
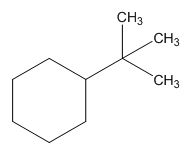
Explanation of Solution
Calculate HDI:
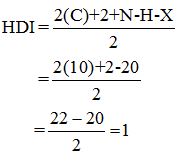
The HDI calculation led to confirm about the presence of a double bond or a ring.
Interpret the given 13CNMR spectrum:
Broadband - decoupled spectrum:
The spectrum shows six signals whereas the given molecular formula also has ten carbon atoms. Thus all the ten carbons have chemically different electronic environments showing signals.
i) The two signals in the region of 0-50ppm indicate the sp3 hybridized carbon atoms which can be methyl / methylene groups.
DEPT -90: Spectrum has signals led to confirmation of CH group in the structure.
DEPT -135 (gives signals of CH2, C, CH3 groups):
ii) The two positive signals indicate the presence of three methyl groups as no signals appear in the DEPT -90 spectrum.
iii) The three negative signals indicate the presence of five methylene groups; only methylene groups appear negative in the spectrum.
iv) So far the group of fragments obtained is
Two -CH3, -CH2
From the fragments (CH3)3 + (CH2)5 + CH + C = 20 protons) twenty protons are obtained whereas the total number of protons from the molecular formula is also twenty.
The structure of a given molecular formula C10H20 is predicted using 13CNMR spectra.
d)
Interpretation:
The structure of an given molecular formula C8H16 to be predicted using 13CNMR spectra.
Concept introduction:
The 13CNMR spectrum gives information on the different electronic environments of carbon. As like 1HNMR, the number of signals generated in 13CNMR are predicted by performing symmetry operations (rotation or reflection symmetry). Only chemical shift values are reported in the spectrum but not the multiplicity and integration values because the coupling between two neighboring 13C - 13C nuclei are weakly involved due to the low abundance of 13C isotopes of carbon atom.
Broadband-decoupled 13CNMR spectrum: The spectra provide information regarding the total number of carbon environments.
DEPT (Distortionless enhancement by polarization transfer):
i) DEPT -90: The spectrum exhibits signal only from CH group and no signals from CH3, CH2, CH and quaternary carbon (carbon with no protons).
ii) DEPT -135: The spectrum exhibits CH3 groups and CH groups as positive signals (pointing up); CH2 groups appear as negative signals (pointing down) and quaternary carbon does not appear.
The signals appear in each type of spectrum:

To identify:
The structure of given molecular formula C6H10.
Answer to Problem 47AP
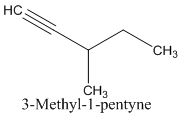
Explanation of Solution
Calculate HDI:
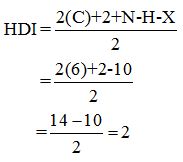
The HDI calculation led to confirm about the presence of a double bond or a ring. (two unsaturated degree).
Interpret the given 13CNMR spectrum:
Broadband - decoupled spectrum:
The spectrum shows six signals whereas the given molecular formula also has six carbon atoms. Thus all the six carbons have chemically different electronic environments showing signals.
i) The two signals in the region of 0-50ppm indicate the sp3 hybridized carbon atoms which can be methyl / methylene groups.
DEPT -90: Spectrum has signals led to confirmation of CH group in the structure.
DEPT -135 (gives signals of CH2, C, CH3 groups):
ii) The four positive signals indicate the presence of two methyl groups as no signals appear in the DEPT -90 spectrum.
iii) The negative signals indicate the presence of methylene groups; only methylene groups appear negative in the spectrum.
iv) So far the group of fragments obtained is
Two -CH3, -CH2
From the fragments (CH3)2 + CH2 + (CH)2 + C = 10 protons) ten protons are obtained whereas the total number of protons from the molecular formula is also ten.
The structure of a given molecular formula C6H10 is predicted using 13CNMR spectra.
e)
Interpretation:
The structure of an given molecular formula C8H16 to be predicted using 13CNMR spectra.
Concept introduction:
The 13CNMR spectrum gives information on the different electronic environments of carbon. As like 1HNMR, the number of signals generated in 13CNMR are predicted by performing symmetry operations (rotation or reflection symmetry). Only chemical shift values are reported in the spectrum but not the multiplicity and integration values because the coupling between two neighboring 13C - 13C nuclei are weakly involved due to the low abundance of 13C isotopes of carbon atom.
Broadband-decoupled 13CNMR spectrum: The spectra provide information regarding the total number of carbon environments.
DEPT (Distortionless enhancement by polarization transfer):
i) DEPT -90: The spectrum exhibits signal only from CH group and no signals from CH3, CH2, CH and quaternary carbon (carbon with no protons).
ii) DEPT -135: The spectrum exhibits CH3 groups and CH groups as positive signals (pointing up); CH2 groups appear as negative signals (pointing down) and quaternary carbon does not appear.
The signals appear in each type of spectrum:

To identify:
The structure of given molecular formula C8H16.
Answer to Problem 47AP
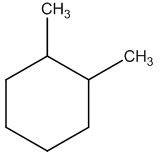
Explanation of Solution
Calculate HDI:
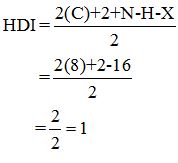
The HDI calculation led to confirm about the presence of a double bond or a ring.
Interpret the given 13CNMR spectrum:
Broadband - decoupled spectrum:
The spectrum shows four signals whereas the given molecular formula also has eight carbon atoms. Thus all the eight carbons have chemically different electronic environments showing signals.
i) The four signals in the region of 0-50ppm indicate the sp3 hybridized carbon atoms which can be methyl / methylene or Methine groups.
DEPT -90: Spectrum has no signals led to confirmation of CH group in the structure.
DEPT -135 (gives signals of CH2, CH3 and CH groups):
ii) The two positive signals indicate the presence of two methyl groups as no signals appear in the DEPT -90 spectrum.
iii) The two negative signals indicate the presence of four methylene groups; only methylene groups appear negative in the spectrum.
iv) So far the group of fragments obtained is
Two -CH3, -CH-, four -CH2
From the fragments (CH3 + CH3 + (CH2)4 + (CH)2 = 16 protons) sixteen protons are obtained whereas the total number of protons from the molecular formula is also sixteen.
The structure of a given molecular formula C8H16 is predicted using 13CNMR spectra.
f)
Interpretation:
The structure of an given molecular formula C8H16 to be predicted using 13CNMR spectra.
Concept introduction:
The 13CNMR spectrum gives information on the different electronic environments of carbon. As like 1HNMR, the number of signals generated in 13CNMR are predicted by performing symmetry operations (rotation or reflection symmetry). Only chemical shift values are reported in the spectrum but not the multiplicity and integration values because the coupling between two neighboring 13C - 13C nuclei are weakly involved due to the low abundance of 13C isotopes of carbon atom.
Broadband-decoupled 13CNMR spectrum: The spectra provide information regarding the total number of carbon environments.
DEPT (Distortionless enhancement by polarization transfer):
i) DEPT -90: The spectrum exhibits signal only from CH group and no signals from CH3, CH2, CH and quaternary carbon (carbon with no protons).
ii) DEPT -135: The spectrum exhibits CH3 groups and CH groups as positive signals (pointing up); CH2 groups appear as negative signals (pointing down) and quaternary carbon does not appear.
The signals appear in each type of spectrum:

To identify:
The structure of given molecular formula C6H10O.
Answer to Problem 47AP
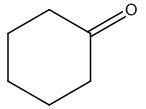
Explanation of Solution
Calculate HDI:
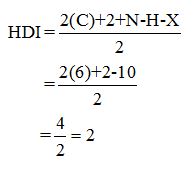
The HDI calculation led to confirm about the presence of a double bond or a ring.
Interpret the given 13CNMR spectrum:
Broadband - decoupled spectrum:
The spectrum shows four signals whereas the given molecular formula also has six carbon atoms. Thus all the six carbons have chemically different electronic environments showing signals.
i) The four signals in the region of 0-50ppm indicate the sp3 hybridized carbon atoms which can be methyl / methylene or Methine groups.
DEPT -135 (gives signals of CH2, C groups):
ii) The two negative signals indicate the presence of four methylene groups; only methylene groups appear negative in the spectrum.
iii) So far the group of fragments obtained is
C=O, -C-, four -ch2
From the fragments (CH2)5 + C = 10 protons) ten protons are obtained whereas the total number of protons from the molecular formula is also ten.
The structure of a given molecular formula C6H10O is predicted using 13CNMR spectra.
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- 3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardAddition of m-xylene to the strongly acidic solvent HF/SbF5 at 45C gives a new species, which shows 1H-NMR resonances at 2.88 (3H), 3.00 (3H), 4.67 (2H), 7.93 (1H), 7.83 (1H), and 8.68 (1H). Assign a structure to the species giving this spectrum.arrow_forwardKetones undergo a reduction when treated with sodium borohydride, NaBH4. What is the structure of the compound produced by reaction of 2-butanone with NaBH4 if it has an IR absorption at 3400 cm-1 and M+=74 in the mass spectrum?arrow_forward
- Following is a 1H-NMR spectrum of2-butanol, Explain why the CH2 protons appear as a complex multiplet rather than as a simple quintet.arrow_forwardGrignard reagents undergo a general and very useful reaction with ketones. Methylmagnesium bromide, for example, reacts with cyclohexanone to yield a product with the formula C7H14O. What is the structure of this product if it has an IR absorption at 3400 cm-1?arrow_forwardIn the 1H NMR spectra of 2-bromopropane (CH3)2CHBr and 1-bromopropane CH3CH2CH2Br, how many signals do you expect to see?arrow_forward
- Analyse the following 13C NMR spectrum of the (R,R)-N,N’-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine compound (structure provided below) and explain.arrow_forwardTreatment of 2-methylpropanenitrile [(CH3)2CHCN] with CH3CH2CH2MgBr, followed by aqueous acid, affords compound V, which has molecular formula C7H14O. V has a strong absorption in its IR spectrum at 1713 cm−1, and gives the following 1H NMR data: 0.91 (triplet, 3 H), 1.09 (doublet, 6 H), 1.6 (multiplet, 2 H), 2.43 (triplet, 2 H), and 2.60 (septet, 1 H) ppm. What is the structure of V? We will learn about this reaction in Chapter 20.arrow_forwardAlcohols undergo an oxidation when treated with Dess-Martin periodinane in dichloromethane. What is the structure of the product of the following reaction if it has an IR absorption at 1715 cm-1and M+ = 86 in the mass spectrum?arrow_forward
- You have an unknown with an absorption at 1680 cm-1; it might be an amide, an isolated double bond, a conjugated ketone, aconjugated aldehyde, or a conjugated carboxylic acid. Describe what spectral characteristics you would look for to help youdetermine which of these possible functional groups might be causing the 1680 peak.arrow_forwardHow many signals would you expect to see in the 13C NMR and 1H NMR spectra of cis-1,3- dimethylcyclohexane:arrow_forwardThe following compound has a molecular formula of C5H10O and the indicated 13C spectrum. A proton NMR was measured, but the data was lost except for the fact that it showed three distinct signals: a singlet, a doublet, and a septet, all of which appear between 0 and 3 ppm. Identify the compound, and draw its expected 1H NMR.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


