
Interpretation:
In which rule expect for the
Concept Introduction:
Nitrogen rule: The nitrogen rule states, that a molecule that has no or even number of nitrogen atoms has an even nominal mass, whereas a molecule that has a odd number of nitrogen atoms has an odd nominal mass.
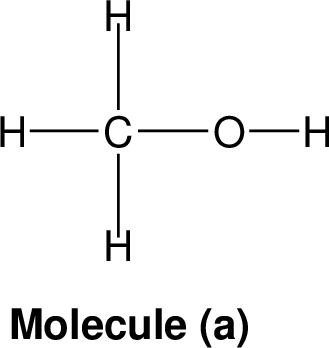 | |
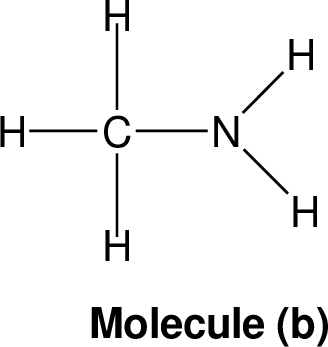 |
Nominal mass: The nominal mass for an element is the mass number of it is most abundant naturally occurring stable isotope and for an ion or given molecule the nominal mass is the sum of the nominal masses of the constituent atoms.
Example: The exact mass of the most abundant
For example hydrogen
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
- Show the structure of the fragment ions for the mass spectrum of ethyl benzoate.arrow_forwardChemistry eed structures for molecular ion fragments and i neef fragmentation pattern predictions pleasearrow_forwardTrue or False 1. Sample preparation of infrared spectroscopy uses KBr as the blank because Kbr has 100% transmittance at the IR wavelength range. 2. The electronegativity difference present in a dipole moment within a bond is directly proportional to the electromagnetic field produced. 3. The electromagnetic radiation that is emitted in infrared spectroscopy is not enough to excite electrons to an unoccupied molecular orbital.arrow_forward
- how will delta e for an isolated c13 nucleus compared with that of a h1 nucleus?arrow_forward50 uL of an aqueous sample of double-stranded DNA is dissolved in 950 uL of water. This diluted solution has a maximal absorbance of 0.326 at 260 nm. What is the concentration of the original (more concentrated) DNA sample, expressed in ug/uL?arrow_forwardDraw structures for the two fragment ions of highest mass from the following molecule.arrow_forward
- What are the pros and cons of HCD vs CID ion fragmentation in mass spectrometry? Is one preferred over another?arrow_forwardHow do you call the small peak usually found at the the left of the moelcular ion peak in a mass spectra? fragment peak relative abundance peak M+1 peak or isotope peak parent peak or molecular ion peakarrow_forwardCan IR spectroscopy be used for quantitative analysis? >No, because IR spectroscopy only records vibrational frequency of bonds. >No, because IR spectroscopy only identifies the types of covalent bonds. >Yes, because the transmittance of the analyte is proportional to concentration. >Yes, because the absorbance of the analyte is proportional to concentration.arrow_forward
- For each of the following molecules, draw the most likely molecular ions (always a radical cation) that you would expect to be formed in an EI-MS experiment. For each case, calculate the m/z value for the molecular ion on the most abundant isotopomer. (C = 12, N = 14, O = 16, Cl = 35, Br = 79)arrow_forwardShich will have no effect on an Rf value in TCL? the molecular weight of the sample. the hydrogen bonding capability of the stationary phase. the polarity of the sample. the polarity of the eluent.arrow_forwardIdentify the remaining three fragments from the table. (m/z=22,16,12) (hint: for m/z=22, the value of Z isn't necessarily 1)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
