
Concept explainers
Propose a structure consistent with each set of data.
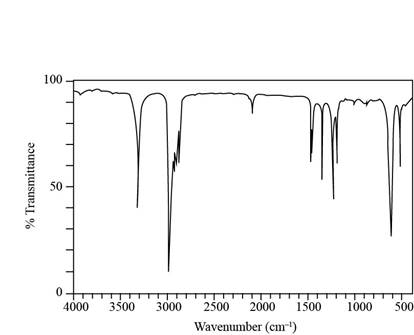
a. A compound X (molecular formula
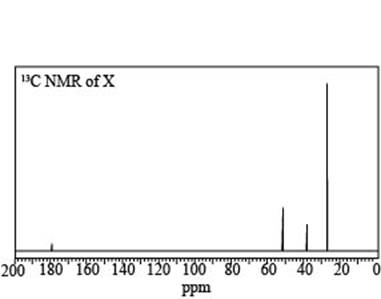
b. A compound Y (molecular formula
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
EBK INTRODUCTION TO CHEMISTRY
Chemistry: Structure and Properties (2nd Edition)
Chemistry: Matter and Change
Introduction to Chemistry
Organic Chemistry As a Second Language: Second Semester Topics
- Propose a structural formula for each compound consistent with its 1H-NMR and 13C-NMR spectra. (a) C5H10O2 (b) C7H14O2 (c) C6 H12O2 (d) C7H12O4 (e) C4H7ClO2 (f) C4H6O2arrow_forwardThe 1H and 13C NMR spectra below belong to a compound with formula C6H10O2. Propose a structure for this compound.arrow_forwardFollowing are 1H-NMR spectra for compounds G, H, and I, each with the molecular formula C5H12O. Each is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas. (a) Propose structural formulas of compounds G, H, and I. (b) Explain why there are four lines between 0.86 and 0.90 for compound G. (c) Explain why the 2H multiplets at 1.5 and 3.5 for compound H are so complex.arrow_forward
- 3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardThe proton NMR spectrum for a compound with formula C10H12O2 is shown below. The infrared spectrum has a strong band at 1711 cm-1. The broadband-decoupled 13C NMR spectral results are tabulated along with the DEPT135 and DEPT90 information. Draw the structure of this compound.arrow_forwardA ¹H NMR spectrum is shown for a molecule with the molecular formula of CsH10O2. Draw the structure that best fits this data. 10 pom aarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


