
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept-Introduction:
Lewis structure
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
(a)
Explanation of Solution
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
Outer valence electrons of Selenium and Florine are six and seven respectively.
Draw Lewis structure of Selenium tetrafluoride:
The Lewis structure of Selenium tetrafluoride follows as,
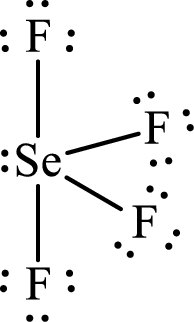
After the distribution of electrons, Selenium atom gets a lone pair of electrons.
Selenium has
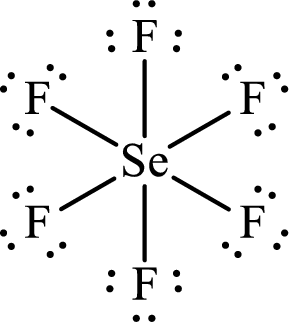
Draw Lewis structure of Selenium hexafluoride:
Selenium has
The Lewis structure of Selenium hexafluoride follows as,
(b)
Interpretation:
Change in orbital hybridization of the central
Concept-Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Geometry of a molecule can be predicted by knowing its hybridization.
(b)
Explanation of Solution
Selenium tetrafluoride and Fluorine reacts to form Selenium hexafluoride.
The geometry of
In
In
During the reaction the hybridization of Selenium changes from
Want to see more full solutions like this?
Chapter 14 Solutions
CHEM 211: CHEMISTRY VOL. 1
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





