
Concept explainers
(a)
Interpretation:
Structure of the 3-hexanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as
Answer to Problem 39P
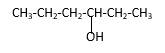
Explanation of Solution
Structure of the 3-hexanol contains six carbon length main carbon chain which connects to an alcohol group in the 3rd carbon of the main carbon chain. There are no sub groups which connect to it. And according to the structure it should be a secondary alcohol.
According to the name, structure of the compound is as below;
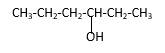
(b)
Interpretation:
Structure of the propyl alcohol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
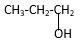
Explanation of Solution
Structure of the propyl alcohol consist one main C chain which contains three C atoms, and alcohol group is connected to the 3rdposition of the main C chain. As per the name it should be a primary alcohol.
Structure of the compound is as below;
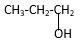
(c)
Interpretation:
Structure of the 2-methylcyclopropanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
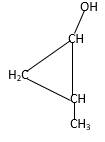
Explanation of Solution
Structure of the 2-methylcyclopropanol consist one main C ring which contains three C atoms, and alcohol group is connected to the 1stposition of the main C ring. Methyl groupis connected to the2nd position of the main C ring. And as per the name it should be a primary alcohol.
Structure of the compound is as below;
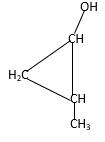
(d)
Interpretation:
Structure of the 1,2-butanediol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
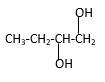
Explanation of Solution
Structure of the 1,2-butanediol consist one main C chain which contains four C atoms, and alcohol groupsare connected to the 2ndand 1stpositions of the main C chain.
Structure of the compound is as below;

(e)
Interpretation:
Structure of the 4,4,5-trimethyl-3-heptanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
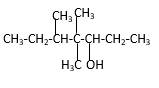
Explanation of Solution
Structure of the 4,4,5-trimethyl-3-heptanol consist one main C chain which contains seven C atoms, and alcohol group is connected to the 3rdposition of the main C chain. Out of three methyl groups, twoare connects to the 4th position of the main C chain and one is connected to the 5th position of the main carbon chain. And as per the name it should be a secondary alcohol.
Structure of the compound is as below;

(f)
Interpretation:
Structure of the 3,5-dimethyl-1-heptanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
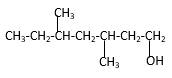
Explanation of Solution
Structure of the 3,5-dimethyl-1-heptanol consist one main C chain which contains seven C atoms, and alcohol group is connected to the 1st position of the main C chain. Methyl groupsare connected to the 3rd and 5th positions of the main C chain. And as per the name it should be a primary alcohol.
Structure of the compound is as below;

Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
- Give the structure corresponding to each IUPAC name. 2,4 dimethyl- 2 hexanolarrow_forward9. What is the IUPAC of the following compound?(CH3CH2)3COH a) 2-Ethyl-2-pentanolb) 3-Ethyl-3-pentanolc) 2-Ethyl-3-pentanold) 2,2-Diethyl-1-butanolarrow_forwardWhat is the IUPAC name of the following compound? a) 2,2-dimethyl-4-butanol b) 3,3-dimethyl-1-butanol c) 1-tert-butyl-2-ethanol d) 2-sec-butyl-1-ethanolarrow_forward
- Draw the structure for each alcohol. a. 2-butanol b. 2-methyl-1-propanol c. 3-ethyl-1-hexanol d. 2-methyl-3-pentanolarrow_forwardIdentify the IUPAC name of the given structure. A. 2 - methylhexan-5-one B. 5 - methylhexan-2-one C. 2 - heptanone D. 5 - heptanone Identify the IUPAC name of the given structure. A. 4 - bromopentan-3-one B. 1 - bromobutan-2-one C. 2 - bromobutan-one D. None of the abovearrow_forwardWhich of the following alcohols is least likely to be soluble in water? 3-pentanol 2-butanol 1,2-ethanediol 1,2,3-propanetriol Methanolarrow_forward
- Draw structures for the four constitutional isomers of molecular formula C4H10O that contain an OH group. Give the IUPAC name for each alcohol.arrow_forwardGive both the IUPAC name and the common name for each alcohol.(a) CH3CH2CH(OH)CH3arrow_forwardFill in the blanks; ______ has a higher boiling point than _________? a. Carbon dioxide; 1-butanamine b. Butane; 2-butanol c. None of these are correct d. 1-methoxypropane; 2-heptene e. Cyclohexane; 1-propanolarrow_forward
- Classify the following alcohols as primary, secondary, or tertiary. 3-ethyl-1-pentanol 5-butyl-4-chloro-5-decanol sec-heptyl alcohol iso-pentyl alcohol 3,3-diethyl-4-nitrononan-4-olarrow_forwardDraw structures corresponding to the following IUPAC names: (a) 3-Methylbutanal (b) 3-Methylbut-3-enal (c) 4-Chloropentan-2-one (d) Phenylacetaldehyde (e) 2,2-Dimethylcyclohexanecarbaldehyde (f ) Cyclohexane-1,3-dionearrow_forwardIdentify the IUPAC name of the given compound. a) 1-propylbenzophenone b) 1-phenyl-1-propanone c) 1-propylphenylketone d) 1-phenylpropyl ketonearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

