
Concept explainers
(a)
Interpretation:
The dehydrated product formed from the following alcohol with
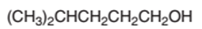
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be a reactant, whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form
Answer to Problem 58P
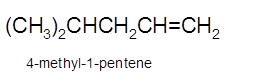
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of 4-methylpentanol will form 4-methyl-1-pentene molecule.
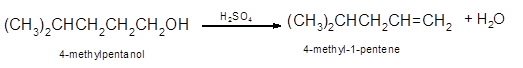
(b)
Interpretation:
The dehydrate product formed from the following alcohol with
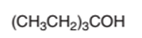
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 58P
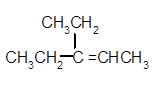
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of given alcohol will form only one alkene as all H next to −OH are the same.
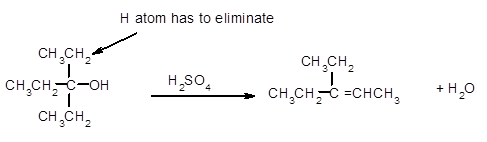
(c)
Interpretation:
The dehydrated product formed from the following alcohol with
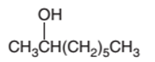
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 58P
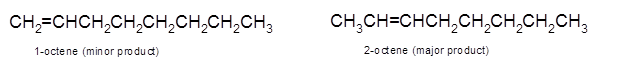
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH groups from two adjacent C's.
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of 2-octanol can form two alkenes as both the neighbor C of OH group have H atoms. Hence, dehydration of 2-octanol will follow Zaitsev rule and will form 2-octene as the major product and 1-octene as a minor product as 2-octene is more substituted alkene than 1-octene.
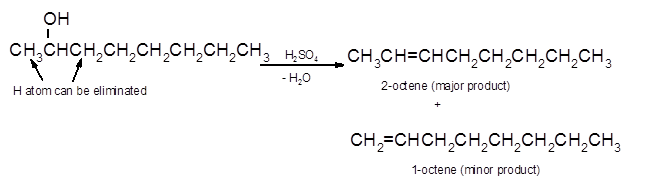
(d)
Interpretation:
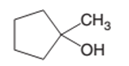
The dehydrate product formed from the following alcohol with
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 58P
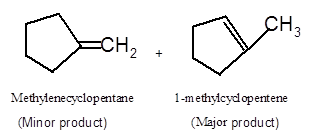
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence the dehydration of given alcohol can form two alkenes as both the neighbor C of OH group have H atoms. Hence dehydration of 1-methylcyclopentanol will follow Zaitsev rule and will form 1-methylcyclpentene as the major product and methylenecyclopentane as a minor product, as 1-methylcyclpentene is more substituted alkene than methylenecyclopentane.
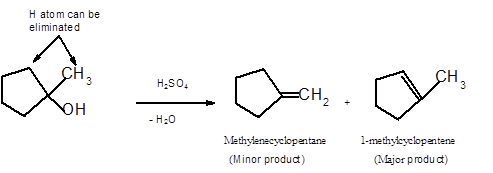
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
- Isopropyl alcohol is Select one: a. CH3CH2OH b. CH3CH(OH)CH3 c. CH3CH2CH2OH d. CH3OHarrow_forwardGive a systematic (IUPAC) name for each diol.(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3 (b) HO¬(CH2)8¬OHarrow_forward1. Which alcohol has a higher boiling point?a. (i) 2-methylpropan-2-ol or (ii) butan-2-olb. (i) hexan-1-ol or (ii) 3,3-dimethylbutan-1-olarrow_forward
- Draw the products formed when each alcohol undergoes dehydration with TsOH, and label the major product when a mixture results.arrow_forwardMolecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forwardDraw the products formed when attached alcohol undergoes dehydration with TsOH, and label the major product when a mixture results.arrow_forward
- Show how each alcohol or diol can be prepared from an alkene. (a) 2-Pentanol (b) 1-Pentanol (c) 2-Methyl-2-pentanol (d) 2-Methyl-2-butanol (e) 3-Pentanol (f) 3-Ethyl-3-pentanol (g) 1,2-Hexanediolarrow_forwardPredict which member of each group is most soluble in water, and explain the reasons for your predictions. phenol, cyclohexanol, or 4-methylcyclohexanolarrow_forwardShow how to prepare each compound from 2-methyl- 1- propanol. a. 2- methylpropene b. 2- methyl- 2- propanol c. 2- methylpropanoic acid (CH3)2CHCOOHarrow_forward
- Draw 2‑methylpropanal. Include all hydrogen atoms. Predict the products when cyclohexanol is heated in the presence of H+.H+. Show all hydrogen atoms.arrow_forward1. which compound is more soluble in water: CH3 (CH2)3 COO- Na+ Or CH3(CH2)3 COOH 2. which compound is more soluble in an organic solvent: CH3 (CH2)3 COO- Na+ Or CH3(CH2)3 COOHarrow_forwardexplain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

