
(a)
Interpretation:
The statement “benzoic acid is an aliphatic carboxylic acid” has to be indicated as true or false. The reason for the false statement has to be described.
(a)
Answer to Problem 6MCP
The given statement is false because benzoic acid is an
Explanation of Solution
Benzoic acid is the simplest aromatic carboxylic acid. It is aromatic due to the presence of a benzene ring in its chemical structure.
The structure of benzoic acid is,
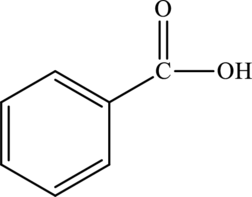
The statement “benzoic acid is an aliphatic carboxylic acid” is false because it is an aromatic carboxylic acid due to the presence of benzene ring.
(b)
Interpretation:
The statement “benzoic acid has a lower boiling point than benzaldehyde” has to be indicated as true or false. The reason for the false statement has to be described.
(b)
Answer to Problem 6MCP
The given statement is false because benzoic acid has higher boiling point than benzaldehyde due to strong intermolecular hydrogen bonding and strong dipole-dipole interactions.
Explanation of Solution
Benzoic acid is a carboxylic acid and benzaldehyde is an aldehyde.
Carboxylic acids have strong intermolecular hydrogen bonding with each other and strong dipole-dipole attractions.
The presence of polar carboxyl group and intermolecular hydrogen bonding makes these acids have higher boiling points. The presence of dimers increases the strength of the van der Waals dispersion forces, which makes them have high boiling points.
Therefore, benzoic acid has higher boiling point than benzaldehyde due to strong intermolecular hydrogen bonding and strong dipole-dipole interactions.
Hence, the statement “benzoic acid has a lower boiling point than benzaldehyde” is false.
(c)
Interpretation:
The statement “benzoic acid reacts with ethanol to form ethyl benzoate” has to be indicated as true or false. The reason for the false statement has to be described.
(c)
Answer to Problem 6MCP
The given statement is true.
Explanation of Solution
Benzoic acid on reaction with ethyl alcohol produces ethyl benzoate and water.
The
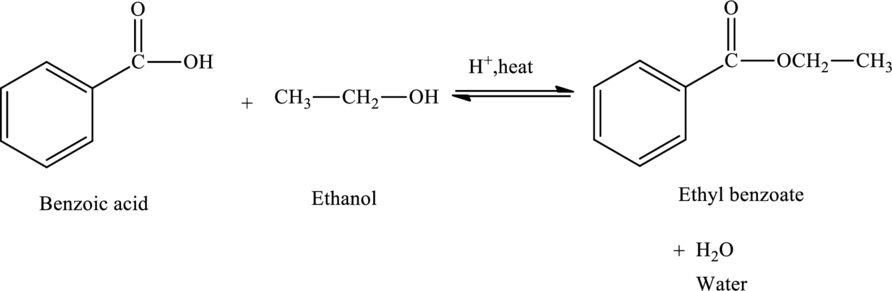
The statement “benzoic acid reacts with ethanol to form ethyl benzoate” is true.
(d)
Interpretation:
The statement “benzoic acid has molecular formula of
(d)
Answer to Problem 6MCP
The given statement is false because the molecular formula of
Explanation of Solution
Benzoic acid is an organic compound with molecular formula of
Hence, the statement “benzoic acid has molecular formula of
(e)
Interpretation:
The statement “benzoic acid is structural isomer of xylene” has to be indicated as true or false. The reason for the false statement has to be described.
(e)
Answer to Problem 6MCP
The given statement is false because the structural isomers of benzoic acids are tropolone, salicyladehyde,
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of benzoic acid
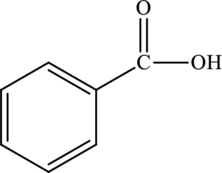
The molecular formula of xylene is
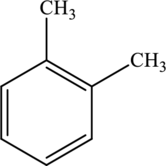
Both these are not structural isomers with each other.
Hence, the statement “benzoic acid is structural isomer of xylene” is false because the structural isomers of benzoic acids are tropolone, salicyladehyde,
(f)
Interpretation:
The statement “benzoic acid is more water soluble in phenol” has to be indicated as true or false. The reason for the false statement has to be described.
(f)
Answer to Problem 6MCP
The given statement is false because phenol is more water soluble than benzoic acid due to its ability to form hydrogen bond with water molecules.
Explanation of Solution
Phenols are water soluble to some extent. This is due to its ability to form hydrogen bonding with water molecules. However, the large part of phenol molecule is the phenyl group that is non-polar and hence its solubility is limited in water.
Whereas, benzoic acid is insoluble in water because of the presence larger nonpolar benzene ring in the structure that cannot be water-soluble.
The statement “benzoic acid is more water soluble in phenol” is false because phenol is more water soluble than benzoic acid due the ability of phenols to form hydrogen bond with water molecules.
(g)
Interpretation:
The statement “sodium benzoate is food preservative and is produced by neutralization of benzoic acid with sodium hydroxide” has to be described as true or false. If the statement is false, the reason that it is false has to be described.
(g)
Answer to Problem 6MCP
The given statement is true.
Explanation of Solution
The reaction of benzoic acid with sodium hydroxide yields sodium salt of benzoic acid and water. The protons of the acid are removed by the
The reaction is,
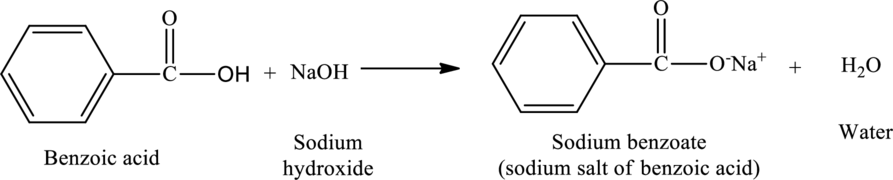
The statement “sodium benzoate is food preservative and is produced by neutralization of benzoic acid with sodium hydroxide” is true.
Want to see more full solutions like this?
Chapter 14 Solutions
GEN ORGANIC CHM LL W/CONNECT
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





