
Concept explainers
(a)
Interpretation:
The product obtained on reaction of
Concept introduction:
Conjugated dienes are defined as two double bonds separated by one single bond. They are more stable than non-conjugated double bonds due to resonance and hybridization energy. Along with that the heat of hydrogenation effects the stability of conjugated dienes. It states that higher the heat of hydrogenation energy lowers the stability of dienes.
Answer to Problem 15.43AP
The complete reaction is written as shown below.
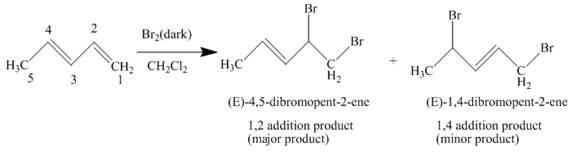
Explanation of Solution
When
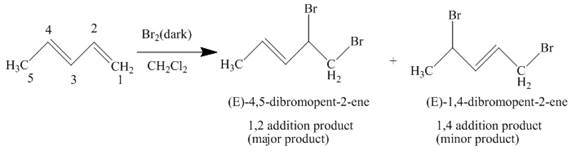
Figure 1
The product obtained on reaction of
(b)
Interpretation:
The product obtained on reaction of
Concept introduction:
Conjugated dienes areb defined as two double bonds separated by one single bond. They are more stable than non-conjugated double bonds due to resonance and hybridization energy. Along with that the heat of hydrogenation effects the stability of conjugated dienes. It states that higher the heat of hydrogenation energy lowers the stability of dienes.
Answer to Problem 15.43AP
The complete reaction is written as shown below.
Explanation of Solution
When

Figure 2
The product obtained on reaction of
(c)
Interpretation:
The product obtained on reaction of
Concept introduction:
Conjugated dienes are defined as two double bonds separated by one single bond. They are more stable than non-conjugated double bonds due to resonance and hybridization energy. Along with that the heat of hydrogenation effects the stability of conjugated dienes. It states that higher the heat of hydrogenation energy lowers the stability of dienes.
Answer to Problem 15.43AP
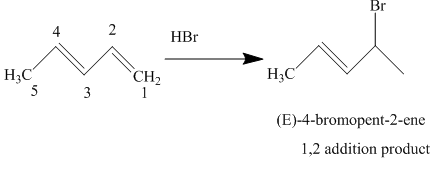
The complete reaction is written as shown below.
Explanation of Solution
When

Figure 3
The product obtained on reaction of
(d)
Interpretation:
The product obtained on reaction of
Concept introduction:
Conjugated dienes are defined as two double bonds separated by one single bond. They are more stable than non-conjugated double bonds due to resonance and hybridization energy. Along with that the heat of hydrogenation effects the stability of conjugated dienes. It states that higher the heat of hydrogenation energy lowers the stability of dienes.
Answer to Problem 15.43AP
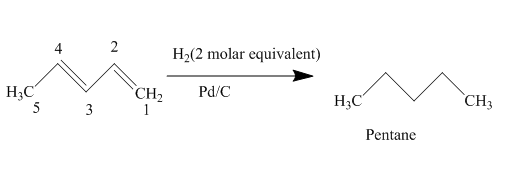
The complete reaction is written as shown below.
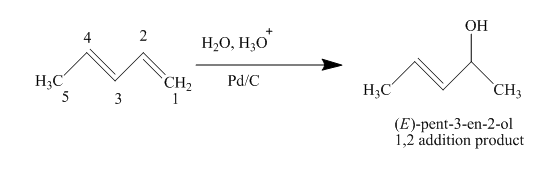
Explanation of Solution
When

Figure 4
The product obtained on reaction of
(e)
Interpretation:
The product obtained on reaction of
Concept introduction:
Conjugated dienes are defined as two double bonds separated by one single bond. They are more stable than non-conjugated double bonds due to resonance and hybridization energy. Along with that the heat of hydrogenation effects the stability of conjugated dienes. It states that higher the heat of hydrogenation energy lowers the stability of dienes.
Answer to Problem 15.43AP
The reaction does not undergo any chemical change because
Explanation of Solution
When
Alkenes do not undergo any nucleophilic addition reaction it means no product is formed in this reaction.
(f)
Interpretation:
The product obtained on reaction of
Concept introduction:
A chemical reaction that involves cycloaddition is known as Diels-Alder reaction. The reactant molecules that give rise to the product are diene and dienophile. The
Answer to Problem 15.43AP
The complete reaction is written below.
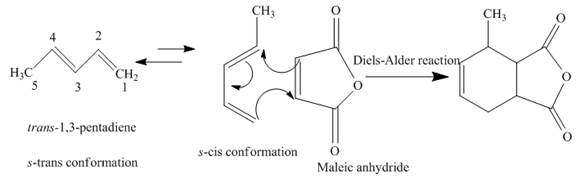
Explanation of Solution
When

Figure 5
The product obtained on reaction of
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- 8a)Provide mechanisms for the following reactions:arrow_forwardReaction of iodoethane with CN- yields a small amount of isonitrile, CH3CH2N≡C, along with the nitrile CH3CH2N≡N, as the major product. Write electron-dot structures for both products, assign formal charges as necessary, and propose mechanisms to account for their formation.arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forward
- In a strongly acidic solution, cyclohexa-1,4-diene tautomerizes to cyclohexa-1,3-diene.Propose a mechanism for this rearrangement, and explain why it is energetically favorable.Parrow_forwardplease provide the mechanisims for the reactions of 1a ,1e,1farrow_forwardWhen 3,3-dimethyl-2-bu tanol is treated with concentrated HI, a rearrangement takes place. Which alkyl iodide would you expectfrom the reaction? Show the mechanism by which it is formed. A. Draw the product of the reaction of 3,3-dimethyl-2-butanol with concentrated HI. B. Write the mechanism for step one of this reaction. Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. C. Write the mechanism for step two of this reaction. Show lone pairs and formal charges. No hydrogen should be shown drawn out with a covalent bond in this step. D. Write the mechanism for step three of this reaction. Show lone pairs and formal charges.NOTE: When drawing mechanistic steps that involve the movement of bonds (such as in a methyl or hydride shift), dotted lines will appear that show which carbon of the bond that you are moving will attack the other group or carbocation. Pay careful attention to those dotted lines when drawing your mechanism. E. Write…arrow_forward
- When 2,2-dibromo-1-phenylpropane is heated overnight with sodium amide at 150 °C, the major product (after addition of water) is a different foul-smelling compound of formula C9H8. Propose a structure for this product, and give a mechanism to account for its formation.arrow_forwardProvide a reasonable arrow-pushing mechanism for Reaction 5b, and explain the the stereochemical outcome. 5d belowarrow_forwardWhich of the following compounds produce the 3 products shown on reaction with KMnO4 and acid (H+). a. I b. II c. III d. II and IIIarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

