
(a)
Interpretation:
Whether the carbocation formed by protonation of isoprene at
Concept introduction:
The delocalization of electrons results in the formation of resonance structure. The curved-arrow notation traces the flow of the electrons in a compound. This notation is used to derive the resonance structure.
Answer to Problem 15.65AP
The carbocation which is formed by protonation of isoprene at
Explanation of Solution
The given structure of isoprene is shown below.
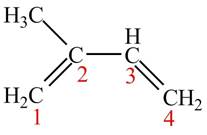
Figure 1
At the time of protonation at carbon
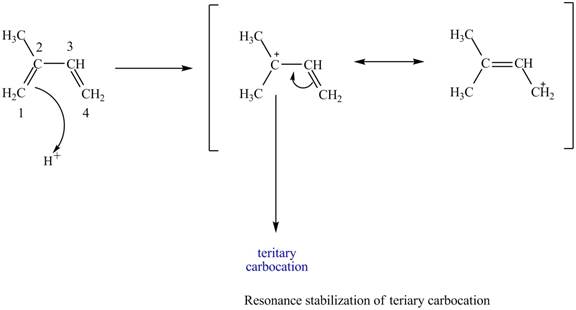
Figure 2
At the time of protonation at carbon
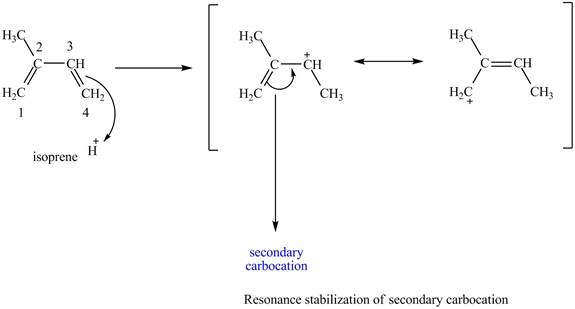
Figure 3
The more stable carbocation is selected on the basis of inductive effect as both carbocation at carbon
Therefore, tertiary carbocation formed at carbon
The carbocation which is formed by protonation of isoprene at
(b)
Interpretation:
The products that are formed by the addition of one equivalent of
Concept introduction:
The delocalization of electrons results in the formation of resonance structure. The curved-arrow notation traces the flow of the electrons in a compound. This notation is used to derive the resonance structure.
Answer to Problem 15.65AP
The products that are formed by the addition of one equivalent of
Explanation of Solution
The given structure of isoprene is shown below.
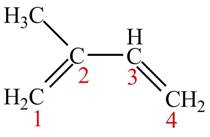
Figure 1
An isoprene always forms the
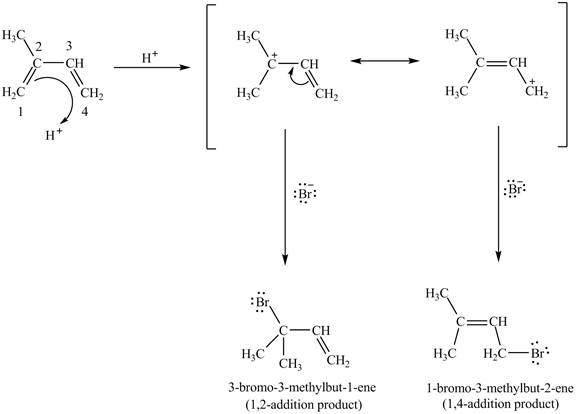
Figure 4
Therefore, the products formed by the conjugated diene, isoprene are
The products,
(c)
Interpretation:
The products that are formed the addition of one equivalent of
Concept introduction:
The delocalization of electrons results in the formation of resonance structure. The curved-arrow notation traces the flow of the electrons in a compound. This notation is used to derive the resonance structure.
Answer to Problem 15.65AP
The products that are formed the addition of one equivalent of
Explanation of Solution
In case of
The protonation of
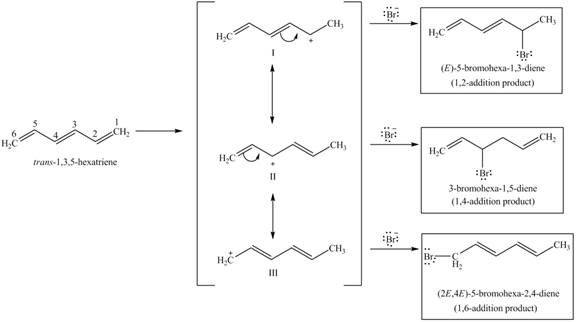
Figure 5
The reaction of
The products,
(d)
Interpretation:
The products which are formed in part (b) and (c) whether kinetically controlled products or
Concept introduction:
In the given conditions of the reaction, if the products of any reaction do not attain the equilibrium then the reaction is known as kinetically controlled reaction.
In the given conditions of the reaction, if the products of any reaction attain the equilibrium then the reaction is known as thermodynamically controlled reaction.
Answer to Problem 15.65AP
In part (b),
In part (c),
Explanation of Solution
The kinetically controlled products are formed much faster than thermodynamically controlled products. So, the
In part (b), the reaction of the given isoprene with
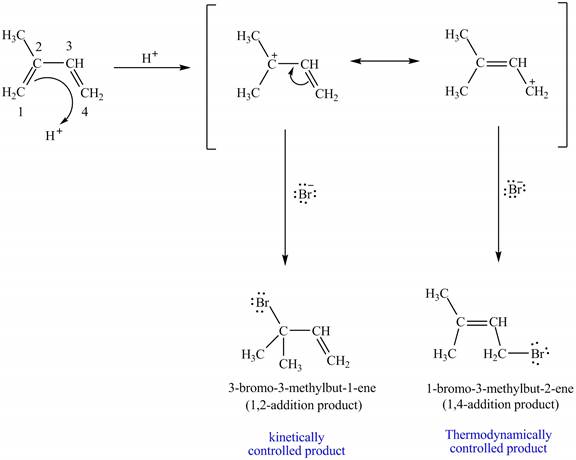
Figure 6
In part (c), the slowest addition is the
Therefore,
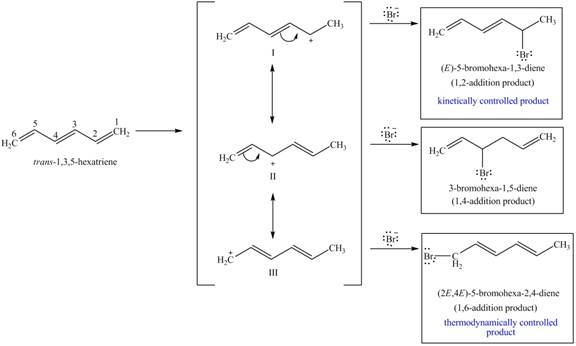
Figure 7
In part (b),
In part (c),
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardSolvolysis of the following bicyclic compound in acetic acid gives a mixture of products, two of which are shown. The leaving group is the anion of a sulfonic acid, ArSO3H. A sulfonic acid is a strong acid, and its anion, ArSO3, is a weak base and a good leaving group. Propose a mechanism for this reaction.arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forward
- Explain mechanism - Base-Catalyzed Addition of H2O to a Carbonyl Group ?arrow_forwardGive all the monobromination products of 2-methylpropane (or isobutane) in presence of heat or energy. Identify the major product and propose a mechanism leading to the formation of the major product. Provide a reaction in the termination step.arrow_forward3b)Give the mechanisms for the following transformations:arrow_forward
- Draw the reaction mechanism of conversion of compound 4a to 4b. Indicate what type of reaction mechanism. Write conditions and reagents used if there are any. Provide brief background in every step of the reaction mechanism.arrow_forwardWrite the reaction mechanism of the following transformations:arrow_forwardAcid-catalyzed dehydration of neopentyl alcohol, (CH3)3CCH2OH, yields 2-methyl-2- butene as the major product. Outline a mechanism showing all steps in its formation.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

