
Interpretation:
The delocalization energy of
Concept introduction:
The term conjugated dienes is used when the double bonds are present alternatively in a hydrocarbon chain. The mathematical function that describes the wave like behavior of an electron in a molecule is expressed by molecular orbital.
The delocalization energy for conjugated molecules is defined as the extra stability that a molecule attains by spreading its electron over the entire molecule. The delocalization of
Answer to Problem 15.2P
The delocalization energy of
Explanation of Solution
The delocalization energy of
The energy of ethylene bonding molecular orbital is
The formula for the calculation of
The bonding molecular orbital (BMOs) of ethylene contains two electrons.
The calculation of
The calculation of
The diagram for orbital interaction in
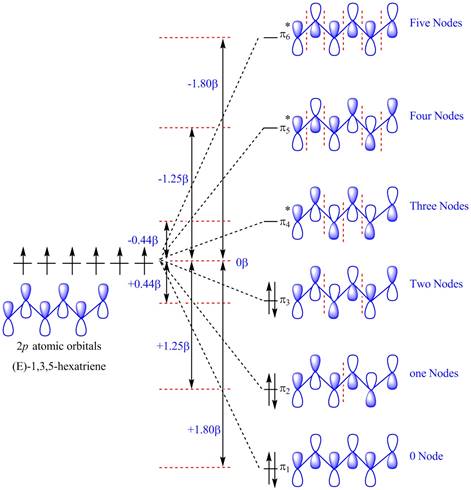
Figure 1
The
The energy of electrons in
The energy of electrons in
The energy of electrons in
Substitute the values of the energies of
Now, substitute the value from equation (3) and (5) in equation (1) in order to calculate the value of delocalization energy of the molecule
Now,
Therefore, the delocalization energy obtained for
The molecule
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- What is the best way to draw a molecular orbital diagram for 1,3-cyclopentadiene, identifying the Lowest unoccupied molecular orbital and the highest occupied molecular orbital?arrow_forwardWhat is the degree of unsaturation of C8H10ClNO? a. 4 b. 5 c. 6 d. 7arrow_forwardAnswer the following questions for 1,3,5- hexatriene, the conjugated triene containing six carbons. Which MOs are the frontier molecular orbitals?arrow_forward
- What is the total number of nodes in the c3 and c4 MOs of 1,3-butadiene?arrow_forwardAnswer the following question for 1,3,5- hexatriene, the conjugated triene containing six carbons. Which MOs are the frontier molecular orbitals?arrow_forward1.The [CoCl4]2- anion is intensely blue colored and [CoCl6]4- is a lightly colored pink. explain breifly 2.Why is the method of descending symmetry needed to derive an mo diagram for acetylene (ethyne), but not needed for diazene((NH)2)?arrow_forward
- Answer the following questions for 1,3,5- hexatriene, the conjugated triene containing six carbons.How many ? -molecular orbitals are there?arrow_forward4 predict the diels alder starting materialarrow_forwardWhat is the delocalization energy and π-bond formation energy of (i) the allyl radical, (ii) the cyclobutadiene cation?arrow_forward
- Give a molecular orbital description for the 1,3,5-heptatriene:arrow_forwardThe degree of unsaturation, or index of hydrogen deficiency, is the number of pi bonds plus rings in a molecule.Specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas:(a) C10H8(b) C8H10O2(c) C8H9NO2arrow_forwardThe Diels-Alder reaction below is very slow. Give two reasons for this observation.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
