
(a)
Interpretation:
The incomplete reaction between
Concept introduction:
The Lindlar’s catalyst is composed of
Answer to Problem 15.80AP
The complete reaction is shown below.
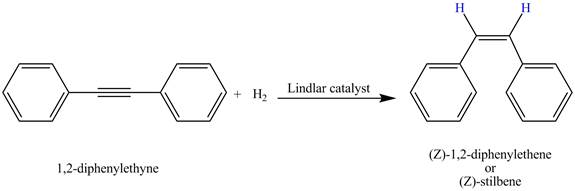
Explanation of Solution
The given incomplete reaction is shown below.
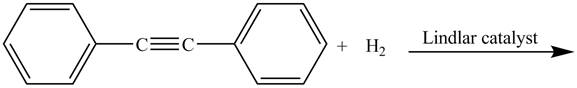
Figure 1
In the above incomplete reaction,
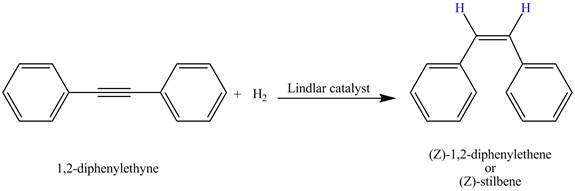
Figure 2
Therefore, the reduced product formed by
The complete reaction corresponding to the given incomplete reaction is shown in Figure 2.
(b)
Interpretation:
The incomplete reaction between diene and dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 15.80AP
The complete reaction is shown below.
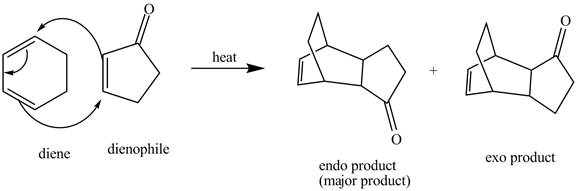
Explanation of Solution
The given incomplete reaction is shown below.
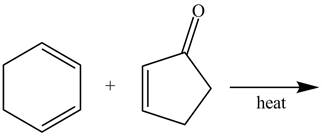
Figure 3
In the above incomplete reaction, a diene undergoes Diels Alder reaction with a dienophile in the presence of heat to form an endo product and an exo product as shown below.
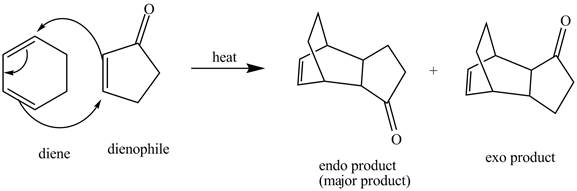
Figure 4
Therefore, two products are obtained from the above shown Diels Alder reaction. One product is the major one that is an endo product and second product which is an exo product is the minor one.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 4.
(c)
Interpretation:
The incomplete reaction between diene and dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 15.80AP
The complete reaction is shown below.
Explanation of Solution
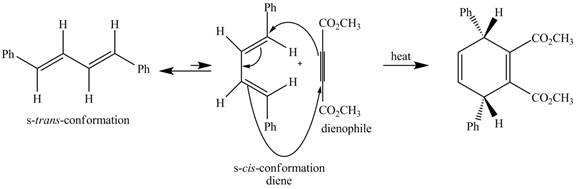
The given incomplete reaction is shown below.
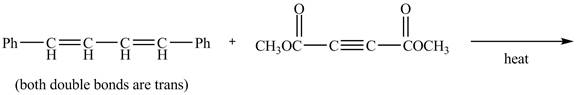
Figure 5
In the above incomplete reaction, the given
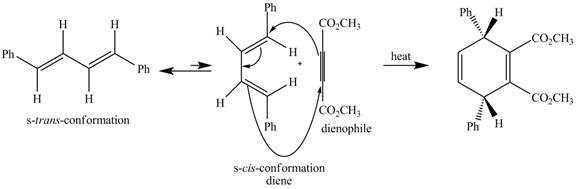
Figure 6
Therefore, one product is obtained from the above shown Diels Alder reaction.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 6.
(d)
Interpretation:
The incomplete reaction between an alkene and benzoquinone is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 15.80AP
The complete reaction is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
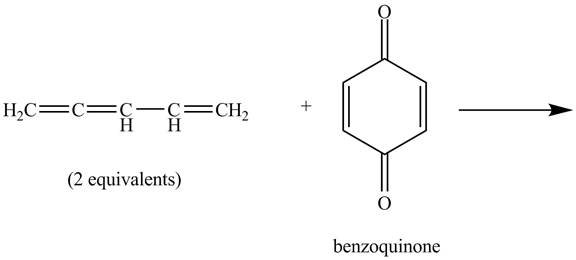
Figure 7
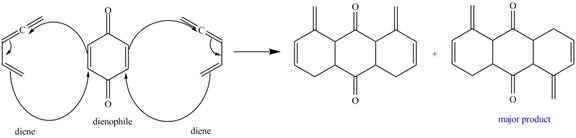
In the above incomplete reaction, the given two equivalents of alkene behaves as a diene and undergoes Diels Alder reaction with benzoquinone which behaves as a dienophile in the presence of heat to form two products as shown below.

Figure 8
Therefore, two products are obtained from the above shown Diels Alder reaction. The second product is the major one because of the less van der Waals repulsion present in between two double bonds which are exocyclic.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 8.
(e)
Interpretation:
The incomplete reaction between a diene and a dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 15.80AP
The complete reaction is shown below.
Explanation of Solution
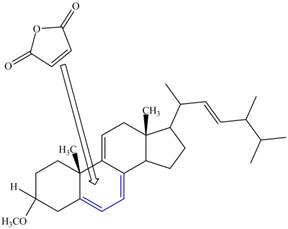
The given incomplete reaction is shown below.
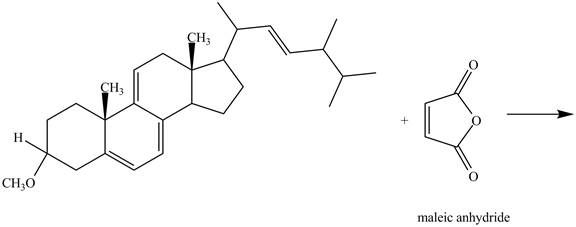
Figure 9
Only the
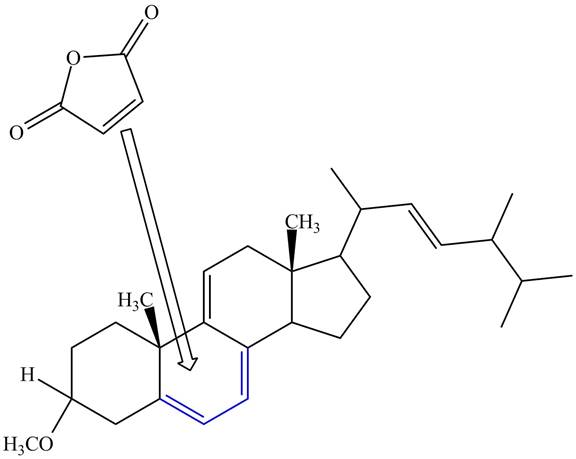
Figure 10
The above reaction does not form any product because the
Therefore, no product is formed in the above shown reaction.
There is no formation of the product takes place in the given reaction.
(f)
Interpretation:
The incomplete reaction between a diene and a dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 15.80AP
The complete reaction is shown below.
Explanation of Solution
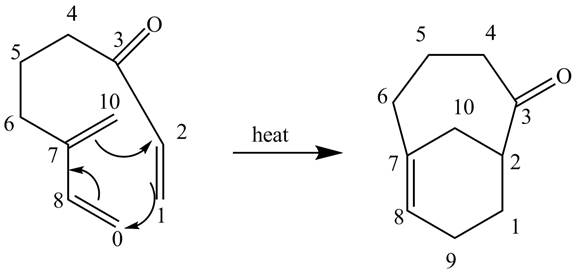
The given incomplete reaction is shown below.
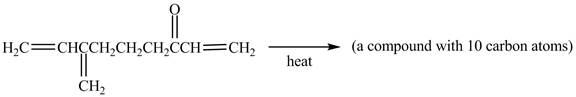
Figure 11
In the above incomplete reaction, the intramolecular Diels Alder reaction takes place in the presence of heat to form two products as shown below.
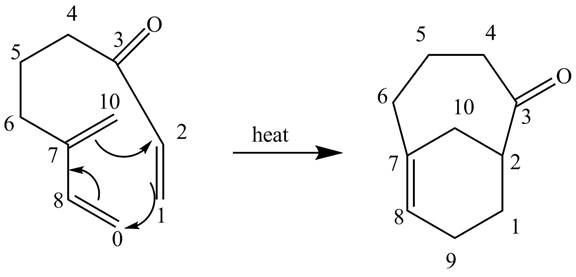
Figure 12
In the above reaction, diene and dienophile are present in the same compound. Therefore, two products are obtained from the above shown Diels Alder reaction. Therefore, the shifting of bonds takes place within the molecule to form a single product.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 12.
(g)
Interpretation:
The incomplete reaction between nickel choride and
Concept introduction:
Metallocene compounds are composed of an electropositive metal ions specially ![]() cyclopentadienyl anion. Metallocenes are the bright colored species.
cyclopentadienyl anion. Metallocenes are the bright colored species.
Answer to Problem 15.80AP
The complete reaction is shown below.
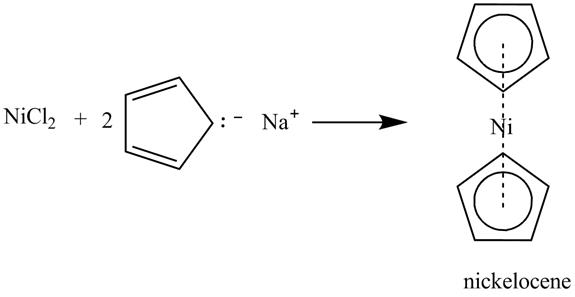
Explanation of Solution
The given incomplete reaction is shown below.
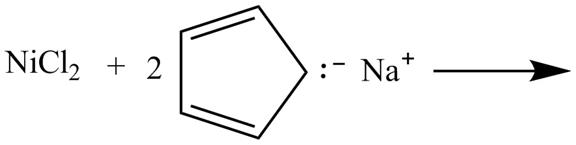
Figure 13
In the above incomplete reaction, the nickel chloride reacts with
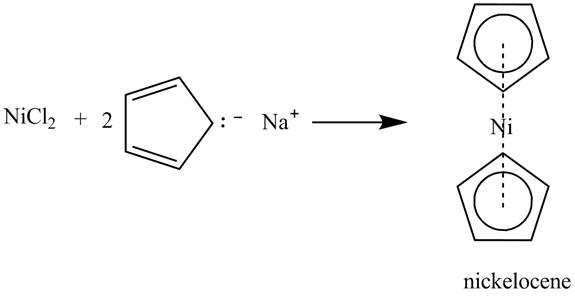
Figure 14
Therefore, the reaction between nickel chloride and
The complete reaction corresponding to the incomplete reaction between nickel choride and
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Give a clear handwritten answer with explanation needed!!! Give the mechanism...arrow_forwardExplain clearly Giving and example and mechanisms, explain the aromatic Substitution reactions.arrow_forwardGive the clear handwritten answer with explanation...give the three possible products .....with major, minor productsarrow_forward
- Give a clear handwritten answer with explanation..give the synthesis in detailedarrow_forwardGive a clear handwritten answer with explanation....give the products when given bleow structure reacts presence of giving each reagents ...arrow_forwardGive a clear handwritten answer with explanation. ..give below some options choose the in which given options who react with Tollen's reagent...give textual explanation also...?arrow_forward
- Give a clear handwritten answer with explanation..give the mechanism of given bleow reaction with detailed answer..?arrow_forwardGive a clear handwritten answer with explanation....complete the following reaction with their stereochemistry....arrow_forwardGive a clear handwritten answer with explanation....give the all stereoisomers with explanation given bleow structurearrow_forward
- Give the machanism of this reactionarrow_forwardGive a clear handwritten answer..give the mechanism with explanation needed!!!arrow_forwardWhich compound (i or ii) is the stronger base? Discuss your answer comprehensively by amoungst other providing an acid base reaction for one of the compounds.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





