
Concept explainers
What is the chemical formula of the inorganic product formed, if any, in each of the reactions in Problem 15-78?
- a. Propanal in the Tollens test
- b. 3-Pentanone in the Tollens test
- c. Methylpropanal in the Benedict’s test
- d. Propanone in the Benedict’s test
(a)
Interpretation:
The inorganic product formed when propanal undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
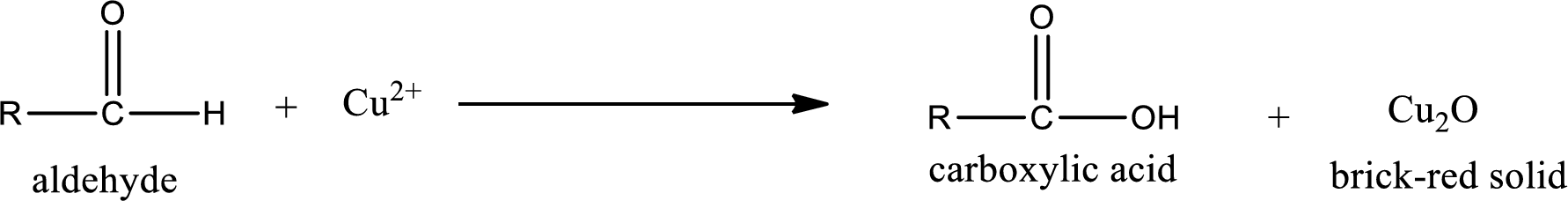
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
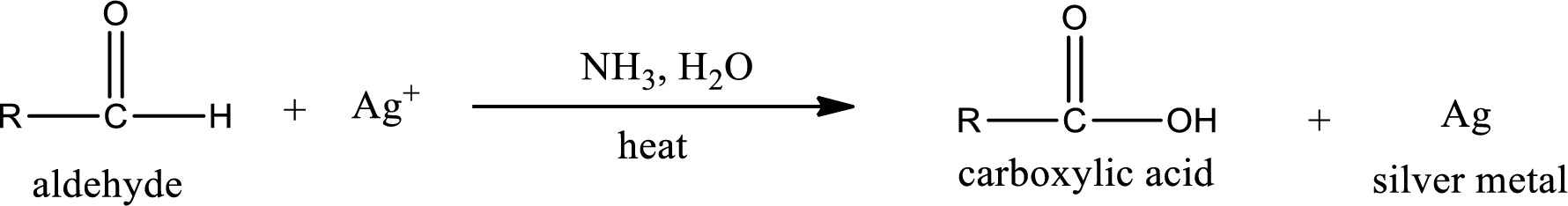
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
The inorganic product formed is silver metal.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
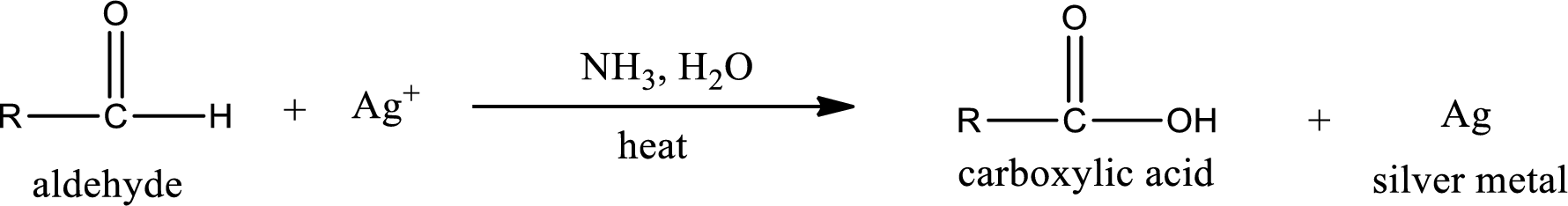
Given aldehyde is propanal and the structure can be given as shown below,
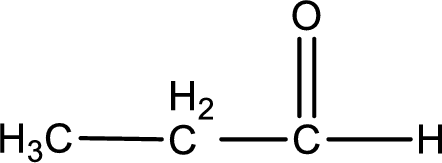
This on reaction with Tollen’s reagent gives carboxylic acid and silver metal as the product. The structure of the inorganic product formed and the complete reaction can be given as shown below,
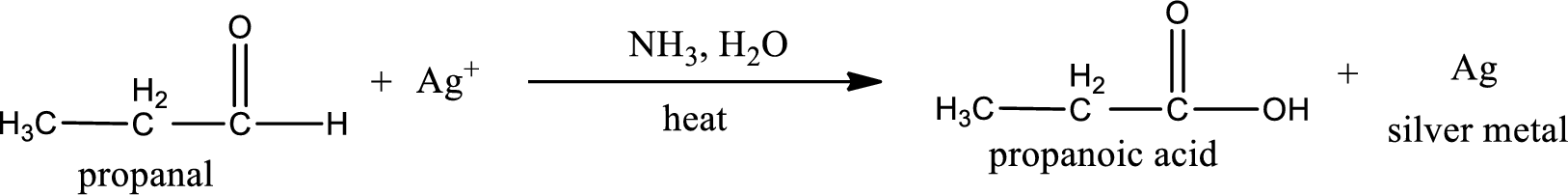
Silver metal is formed as the inorganic product when propanal undergoes Tollen’s test.
The inorganic product formed is given.
(b)
Interpretation:
The inorganic product formed when 3-pentanone undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
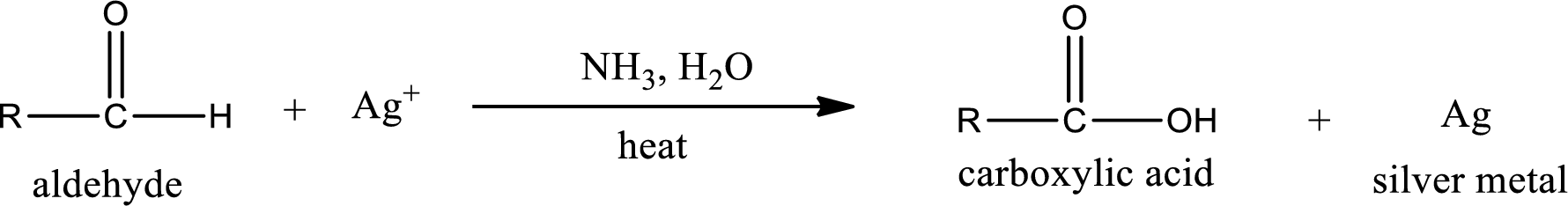
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
No inorganic product is obtained as 3-pentanone does not undergo Tollen’s test.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
Given compound is a ketone that is 3-pentanone and the structure can be given as shown below,
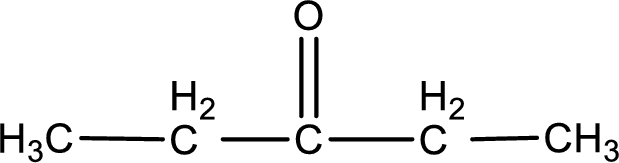
This on reaction with Tollen’s reagent does not give oxidized product. Therefore, no reaction takes place when 3-pentanone reacts with Tollen’s reagent.
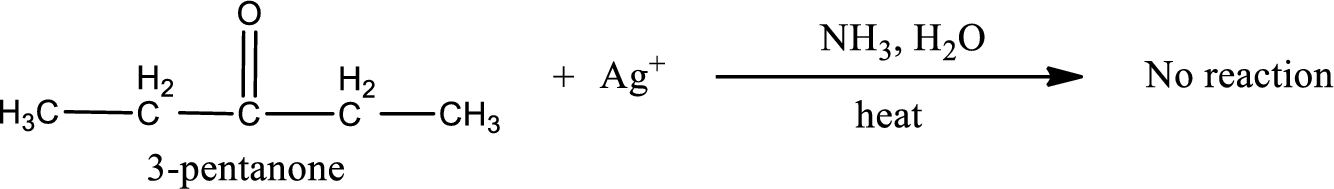
No inorganic product is formed when 3-pentanone undergoes Tollen’s test.
No reaction takes place when 3-pentanone undergoes Tollen’s test.
(c)
Interpretation:
The inorganic product formed when methylpropanal undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
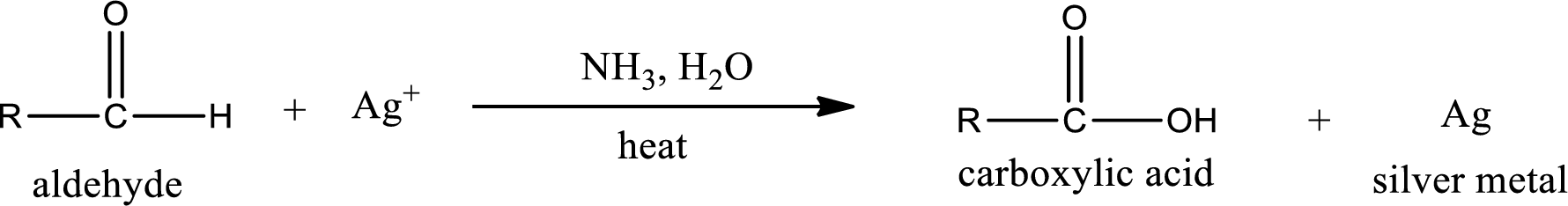
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
The inorganic product formed is
Explanation of Solution
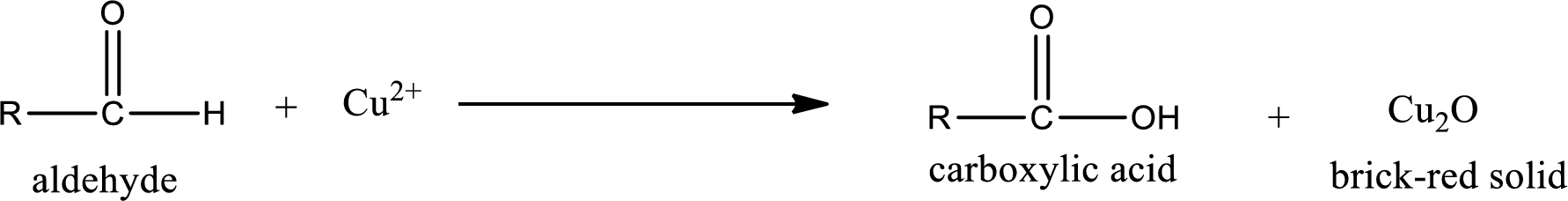
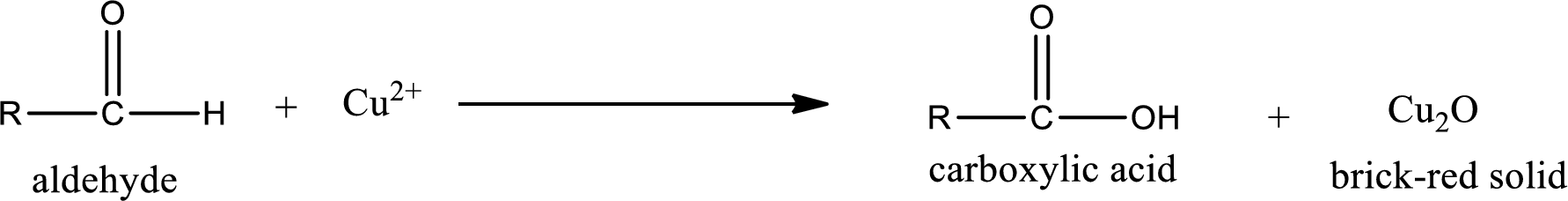
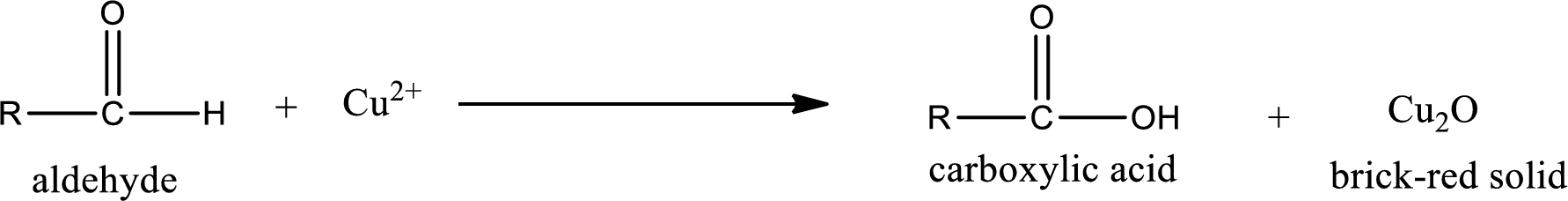
Aldehydes undergo Benedicts’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
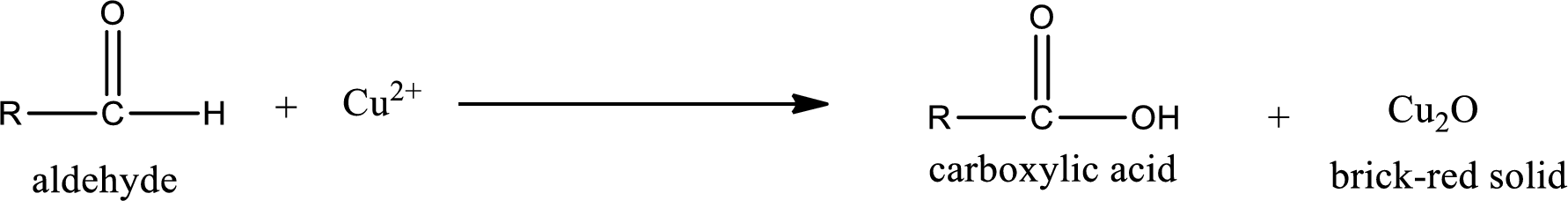
Given aldehyde is methylpropanal and the structure can be given as shown below,
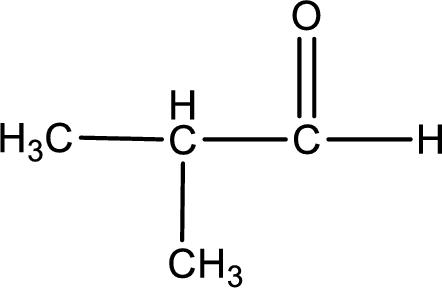
This on reaction with Tollen’s reagent gives carboxylic acid and Copper(I) oxide as the product. The inorganic product formed and the complete reaction can be given as shown below,
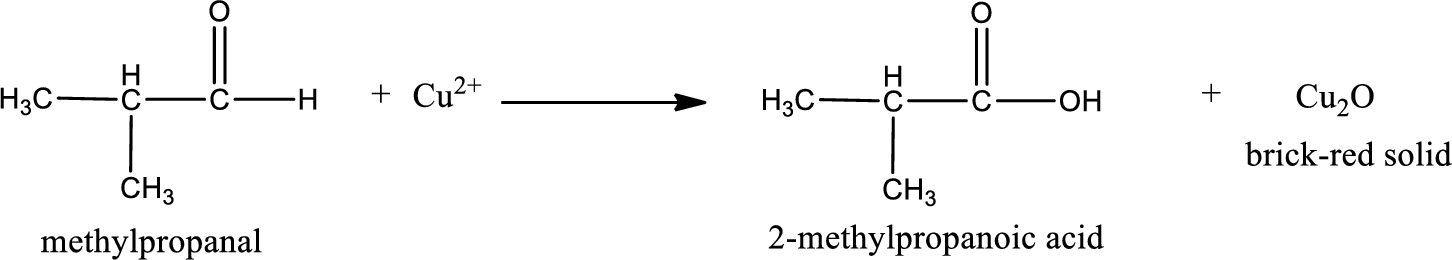
The inorganic product formed when methylpropanal undergoes Benedict’s test is given.
(d)
Interpretation:
The inorganic product formed when propanone undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
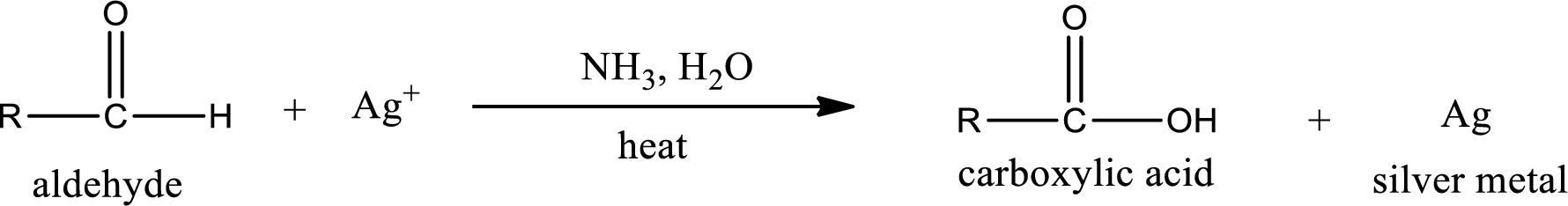
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
No inorganic product is formed when propanone undergoes Benedict’s test.
Explanation of Solution
Aldehydes undergo Benedict’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
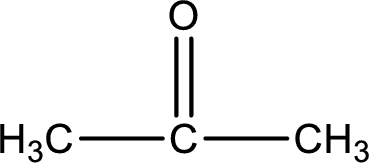
Given compound is a ketone. The name of ketone is propanone and the structure can be given as shown below,
This on reaction with Benedict’s reagent does not give oxidized product. Therefore, no reaction takes place when propanone undergoes Benedict’s test.
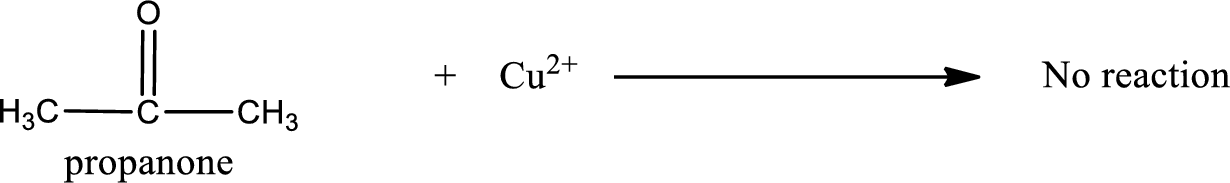
No inorganic product is formed when propanone undergo Benedict’s test.
No reaction takes place when propanone undergoes Benedict’s test.
Want to see more full solutions like this?
Chapter 15 Solutions
Bundle: General, Organic, and Biological Chemistry, Loose-leaf Version, 7th + Study Guide with Selected Solutions + Lab Manual + OWLv2 with Student ... 4 terms (24 months) Printed Access Card
- What were the results of the isotope analysis that was done on this woman’s teeth? A. The iron in the water she drank matched the iron in the water of New Yorkers. B. The oxygen in the water she drank matched the oxygen in the water of New Yorkers. C. The lead in the water she drank matched the lead in the water of Californians. D. The oxygen in the water she drank matched the oxygen in the water of Californians.arrow_forwardChoose the combination of answers that most accurately completes the statement. A chemical with sporicidal properties is a. phenol c. quaternary ammonium compound b. alcohol d. glutaraldehydearrow_forwardhow many hydrogen can be formed from the question abovearrow_forward
- Choose the combination of answers that most accurately completes the statement.The primary mode of action of nonionizing radiation is to a. produce superoxide ions c. denature proteins b. make pyrimidine dimers d. break disulfi de bondsarrow_forwardSome dried beans with a 14C/12C ratio one-eighth of the current value are found in an old cave. How old are the beans?arrow_forwardFill out Table 1 above by writing “+” if the compound tested positive for the chemical reaction. Otherwise, write “-“arrow_forward
- Why is the answer 30 AB/ab; 20 Ab/ab; 20 aB/ab; 30 ab/ab? How do I find that answer and is there a formula I am supposed to use?arrow_forwardWhat element is an essential component in organic compoundsarrow_forwardArachidic acid has a lower melting point than ___________ because the former has a shorter hydrocarbon chain than the latter. A. lignoceric acid B. stearic acid C. palmitic acid D. palmitoleic acid E. arachidonic acidarrow_forward
- Compound X has the above properties. Compound X is a. C2H5OH b. C2H5Cl c. C2H5COOCH3 d. C2H6arrow_forwardMake a conclusion about the qualitative test on lipids You can use this as your reference: https://www.youtube.com/watch?v=2J2t5FRnMtMarrow_forwardWhat is the pH of a solution that has a higher concentration of H+ ions than water? A. > 7 B. 7 C.< 7arrow_forward
 Comprehensive Medical Assisting: Administrative a...NursingISBN:9781305964792Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy CorreaPublisher:Cengage Learning
Comprehensive Medical Assisting: Administrative a...NursingISBN:9781305964792Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy CorreaPublisher:Cengage Learning




