
ORGANIC CHEMISTRY LL >BI<
null Edition
ISBN: 9781260561609
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 26P
A different stereoisomer of
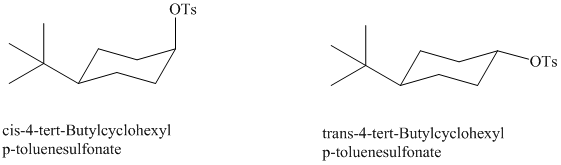
Give the structure of the product from each reactant. One reactant gave a higher yield of the substitution product than the other (
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ignoring stereochemistry, draw the alkylborane formed from the addition of one equivalent of BH3 to the alkene.
The alkylborane formed in Part 1 is further treated with H2O2 and HO−. Draw the two stereoisomers of the final product of the reaction. Include stereochemistry where relevant.
How many stereocenters are formed from the reaction?
What is the relationship between the stereoisomers?
why do (R)-3-chloro-1-methylcycloprop-1-ene and 5-Bromo-1,3-Cyclohexadiene react differently with cyanide (why doesn't cyanide pass through its usual mechanism for these two products and what did you obtain these specific products)
Account for the regioselectivity and stereoselectivity observed when 1-methylcyclopentene is treated with reagent.
Q) Br2 in H2O
Chapter 15 Solutions
ORGANIC CHEMISTRY LL >BI<
Ch. 15.1 - Each of the following organometallic reagents will...Ch. 15.3 - Write equations showing how you could prepare...Ch. 15.4 - Lithium diisopropylamide is often used as a strong...Ch. 15.5 - Write the structure of the organic product of each...Ch. 15.7 - Prob. 5PCh. 15.8 - Prob. 6PCh. 15.9 - Prob. 7PCh. 15.9 - Like nickel, iron reacts with carbon monoxide to...Ch. 15.9 - Prob. 9PCh. 15.9 - What is the oxidation state of manganese in the...
Ch. 15.9 - Prob. 11PCh. 15.10 - Prob. 12PCh. 15.10 - Prob. 13PCh. 15.11 - Give the structure including stereochemistry of...Ch. 15.11 - Prob. 15PCh. 15.12 - Homogeneous catalytic hydrogenation of the...Ch. 15.12 - Prob. 17PCh. 15.13 - What alkenes are formed from 2-pentene by olefin...Ch. 15.13 - The product of the following reaction was isolated...Ch. 15 - Suggest appropriate methods for preparing each of...Ch. 15 - Prob. 21PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 23PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 25PCh. 15 - A different stereoisomer of...Ch. 15 - Prob. 27PCh. 15 - Using phenyllithium and any necessary organic or...Ch. 15 - Prob. 29PCh. 15 - A number of drugs are prepared by reactions in...Ch. 15 - The following conversion was carried out in two...Ch. 15 - Outline syntheses of (a)...Ch. 15 - (S)-(+)-Ibuprofen can be prepared by...Ch. 15 - Like other hydroborations, the reaction of alkynes...Ch. 15 - The sex attractant of the female silkworm has been...Ch. 15 - Prob. 36PCh. 15 - (a) Exaltolide, a musk substance, has been...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and (Cyclobutadiene)tricarbonyliron...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardDescribe the stereochemistry of the bromohydrin formed in each reaction (each reaction is stereospecific). (a) cis-3-Hexene + Br2/H2O (b) trans-3-Hexene + Br2/H2Oarrow_forwardDraw all of the substitution and elimination products formed from the given alkyl halide with each reagent: (a) CH3OH; (b) KOH. Indicate the stereochemistry around the stereogenic centers present in the products, as well as the mechanism by which each product is formed.arrow_forward
- Which stereoisomer of 3-hexene forms (3S,4S)-4-bromo-3-hexanol and (3R,4R)-4-bromo-3-hexanol when it reacts with Br2 and H2O?arrow_forwardIdentify the following pericyclic reaction; explain the course, product distribution and stereochemistry of the reaction. Where the first product is produced 70% and the second product is produced 30%.arrow_forwardWhich of the possible stereoisomers are formed by oxidation of (S )-4-methylcyclohexene with osmium tetroxide? Is the product formed optically active or optically inactive?arrow_forward
- Modify the structure of 3-methyl-1-pentene to show the product formed when it is reacted with: Hydrogen chloride (HCl). Show relevant stereochemistry when applicable. A dilute aqueous solution of sulfuric acid (H2SO4). Show relevant stereochemistry when applicable. Diborane (B2H6) in diglyme, followed by basic hydrogen peroxide (H2O2, OH−). Show relevant stereochemistry when applicable. Bromine (Br2) in water. Show relevant stereochemistry when applicable. Peroxyacetic acid (CH3CO3H). Do not include stereochemistry.arrow_forwardDraw the major products obtained from the reaction of one equivalent of HBr with the following compounds. For each reaction, indicate the kinetic product and the thermodynamic productarrow_forwardwhy do (R)-3-chloro-1-methylcycloprop-1-ene and 5-Bromo-1,3-Cyclohexadiene react differently with cyanide (why doesn't cyanide pass through its usual mechanism for these two products)arrow_forward
- The endiandric acids comprise a group of unsaturated carboxylic acids isolated from a tree that grows in the rainforests of eastern Australia. The methyl esters of endiandric acids D and E have been prepared from polyene Y by a series of two successive electrocyclic reactions: thermal ring closure of the conjugated tetraene followed by ring closure of the resulting conjugated triene. (a) Draw the structures (including stereochemistry) of the methyl esters of endiandric acids D and E. (b) The methyl ester of endiandric acid E undergoes an intramolecular [4 + 2] cycloaddition to form the methyl ester of endiandric acid A. Propose a possible structure for endiandric acid A.arrow_forward1-cyclopentylethan-1-one , H-COH , CH3CH2CHO , CH3CH2OH , 3-pentanone Which ones are soluble in water ? Write the reactions and products.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License