
Cyclobutadiene and (Cyclobutadiene)tricarbonyliron
As we saw in Section
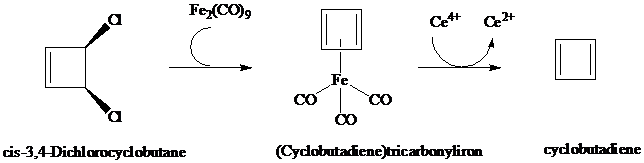
The tricarbonyliron complex of cyclobutadiene is sufficiently stable to undergo reactions
typical of


Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
ORGANIC CHEMISTRY LL >BI<
- Kepone, aldrin, and chlordane are synthesized from hexachlorocyclopentadiene and other five-membered-ring compounds. Show how these three pesticides are composed of two five-membered rings.arrow_forwardProvide a mechanism for the following reaction and rationalise the reactivity in terms of the 3 dimensional structure of the starting material and a consideration of the appropriate orbital interactions.arrow_forwardA conjugated diene with an even number of double bonds undergoes conrotatory ring closure underthermal conditions.arrow_forward
- For the cascade of your choice (A-B-D or A-C-E), explain in detail the reaction mechanism. Considering the Woodward-Hoffmann rules and frontier molecular orbital theory to suggest whether the reaction is allowed or forbiddenarrow_forwardQuestion: How do quantum mechanical effects influence the stability and reactivity of molecules with non-classical carbocations, such as the 2-norbornyl cation, and how does this impact the reaction mechanisms and outcomes?arrow_forwardGive the reaction mechanism and explain in detail. Also perform the woodward Hoffman analysis to explain the selectivity and geometry of the product.arrow_forward
- Discuss the regioselectivity of the compound equation below, including in your answer the terms: radical plusstable, homolytic breakage, main product.arrow_forwardFuran undergoes electrophilic aromatic substitution more readily than benzene; mild reagents and conditions are sufficient.For example, furan reacts with bromine to give 2-bromofuran.(a) Propose mechanisms for the bromination of furan at the 2-position and at the 3-position. Draw the resonance forms ofeach sigma complex, and compare their stabilities.(b) Explain why furan undergoes bromination (and other electrophilic aromatic substitutions) primarily at the 2-position.O Br O123furan 2-bromofuranarrow_forwardCompound A produce compound D while undergo Friedel Crafts Alkylation. Compound D is then oxidized and produce compound E (C11H12O3) as a major product.What are the possible structural formula of compound D and E?arrow_forward
- we know that ethers, such as diethyl ether and tetrahydrofuran, are quite resistant to the action of dilute acids and require hot concentrated HI or HBr for cleavage. However, acetals in which two ether groups are linked to the same carbon undergo hydrolysis readily, even in dilute aqueous acid. How do you account for this marked difference in chemical reactivity toward dilute aqueous acid between ethers and acetals?arrow_forwardIn the early age of the development of Organometallilc Chemistry, the preparation of Organometallic Compound containing M-R(R: alkyl group) bond always led to the decomposition of the M-R bond. It was later known to be caused by the so called “beta-hydrogen elimination” process. Which of the following statements is not correct? This process can be prevented by using all halogenated alkyl group. This process can be prevented by carrying out reaction at high temperature. The Grignard’s reagent, RMgX or R2Mg is stable and does not undergo “b-hydrogen elimination” process. The anti-knocking agent, Pb(C2H5)4 is stable and does not undergo “b-hydrogen elimination” process. The ferrocene, Cp2Fe, is free from the “b-hydrogen elimination” process.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



