
Concept explainers
(a)
Interpretation:
Chirality center of the
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image.
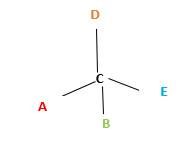
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different
Answer to Problem 30P
In
Explanation of Solution
The
(b)
Interpretation:
Chirality center of the
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image.
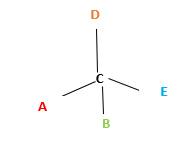
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different functional groups.
Answer to Problem 30P
The compound
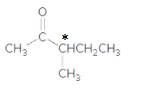
Explanation of Solution
The
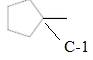
The chiral center is represented as follows:
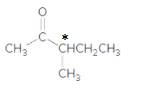
(c)
Interpretation:
Chirality center of the given molecule, should be labeled and identified.
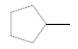
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image.
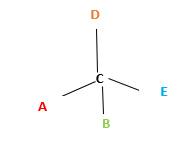
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different functional groups.
Answer to Problem 30P
Inmolecule there are no chiral centers present.
Explanation of Solution
The
Therefore, molecule does not contain any chiral centers.
(d)
Interpretation:
Chirality center of the given molecule, should be labeled and identified.
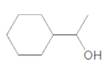
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image.
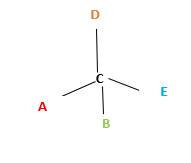
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different functional groups.
Answer to Problem 30P
Inmolecule there is only one chiral center is present. Refer the below mentioned diagram.
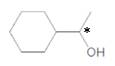
Explanation of Solution
The
C atom which bonded toone
Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
- How are the compounds in each pair related? Are they identical molecules or enantiomers?arrow_forwardOne of the analgesics has a chiral center. Which compound is it? One of the two enantiomers is far more effective at reducing pain than the other.arrow_forwardIdentify the configuration of each chiral center in the following compound Chiral center locations: Configuration(s):arrow_forward
- Determine the relationship between the two drawings: A. Enantiomers B. Same compound C. Constitutional isomers D. Diastereomersarrow_forwardConvert each three-dimensional representation into a Fischer projection.arrow_forwardLocate the stereogenic centers in each compound. A molecule may have one or more stereogenic centers. Gabapentin enacarbil [part (d)] is used to treat seizures and certain types of chronic pain.arrow_forward
- Optically active one compound. Optically active pair of diastereomers. Optically inactive one compound. Optically inactive pair of diastereomers. Optically active pair of enantiomers. Optically inactive pair of enantiomers.arrow_forwardDraw all the possible stereoisomers for each compound, and label pairs of enantiomers and diastereomers.arrow_forwardA(n) ________ is an achiral compound that contains chiral centers but is superimposable on its mirror image. A) constitutional isomers B) conformational isomers C) enantiomers D) diastereomers E) meso compoundsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


