
(a)
Interpretation:
The statement “
(a)
Answer to Problem 3MCP
The given statement is false because amides shows a presence of strong electronegative oxygen atom of carboxyl group causes a very strong attraction between the lone pair of nitrogen electrons and the carbonyl group. Because, of which the unshared pair of electrons cannot hold a proton.
Explanation of Solution
Amide shows a presence of strong electronegative oxygen atom of carboxyl group causes a very strong attraction between the lone pair of nitrogen electrons and the carbonyl group. Because, of which the unshared pair of electrons cannot hold a proton.
Hence,
(b)
Interpretation:
The statement “
(b)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Amides have highest melting point when compared to alcohols and
The ability of primary and secondary amines to form
Hence,
(c)
Interpretation:
The statement “
(c)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(d)
Interpretation:
The statement “
(d)
Answer to Problem 3MCP
The given statement is false because
Explanation of Solution
The structure of
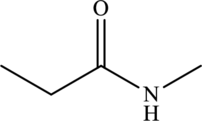
The carbonyl group present in
The statement “
(e)
Interpretation:
The statement “
(e)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
The structure of
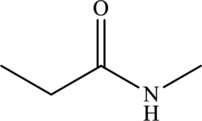
From the structure of
Hence, the given statement is true.
(f)
Interpretation:
The statement “
(f)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Amides have highest melting point when compared to
Hence, the given statement is true.
(g)
Interpretation:
The statement “
(g)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(h)
Interpretation:
The statement “
(h)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(i)
Interpretation:
The statement “
(i)
Answer to Problem 3MCP
The given statement is false because it comprises of carbonyl functional group.
Explanation of Solution
Carboxyl group consists of carbonyl group and hydroxyl group.
The structure of
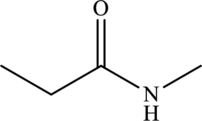
(j)
Interpretation:
The statement “
(j)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of
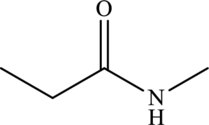
The molecular formula of
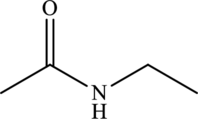
Both these are structural isomers with each other and hence, the statement “
Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





